All Photos(2)
About This Item
Empirical Formula (Hill Notation):
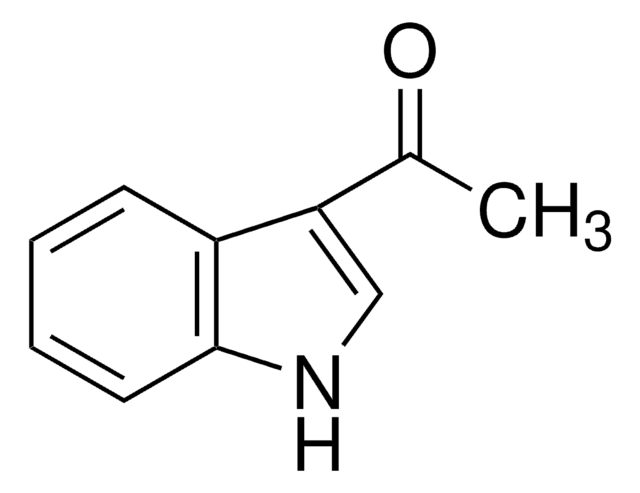
C10H10O
CAS Number:
Molecular Weight:
146.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
70 °C/0.4 mmHg (lit.)
mp
60-62 °C (lit.)
functional group
ketone
SMILES string
Cc1ccc2CCC(=O)c2c1
InChI
1S/C10H10O/c1-7-2-3-8-4-5-10(11)9(8)6-7/h2-3,6H,4-5H2,1H3
InChI key
DBOXRDYLMJMQBB-UHFFFAOYSA-N
General description
6-Methyl-1-indanone is a substituted indanone. It has been synthesized in high quantum yields by the photolysis of α-chloro-2′,5′-dimethylacetophenone. It is formed as one of the photoproduct during the irradiation of 2,5-dimethylphenacyl (DMP) esters. It is reported to be one of the semivolatile component of lamina cigarette smoke.
Application
6-Methyl-1-indanone was used as a starting material for the synthesis of branched alkyl indanes (BINs).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2, 5-Dimethylphenacyl as a new photoreleasable protecting group for carboxylic acids.
Klan P, et al.
Organic Letters, 2(11), 1569-1571 (2000)
Comparisons of Smoke Components in the Semivolatile Phase from Lamina and Midrib Cigarettes of Flue-cured Tobacco Leaves.
Ishiguro S and Sugawara S.
Agricultural and Biological Chemistry, 42(8), 1527-1531 (1978)
Rapid Photochemical Synthesis of 6-Methyl-1-Indanone.
Wang S, et al.
Advanced Materials Research, 634-638, 416-419 (2013)
A laser flash photolysis study of the mechanism of the photocyclization of. alpha.-chloro-ortho-methylacetophenones.
Netto-Ferreira JC and Scaiano JC.
Journal of the American Chemical Society, 113(15), 5800-5803 (1991)
Andrew M Booth et al.
Environmental science & technology, 42(21), 8122-8126 (2008-11-27)
Previously, comprehensive two-dimensional gas chromatography-time of flight-mass-spectrometry (GCxGC-ToF-MS) revealed that the unresolved complex mixtures (UCMs) of contaminant hydrocarbons accumulated by health-affected mussels Mytilus edulis (up to 125 microg g dry weight(-1)) collected from around U.K. coasts, included many isomeric branched
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service