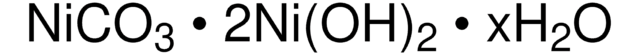377449
Manganese(II) carbonate
≥99.9% trace metals basis
Synonym(s):
Manganese carbonate, Manganese(2+) carbonate, Manganous carbonate
About This Item
Recommended Products
Assay
≥99.9% trace metals basis
form
powder
impurities
≤1,000.0 ppm Trace Metal Analysis
mp
>200 °C (lit.)
solubility
dilute aqueous acid: slightly soluble(lit.)
density
3.12 g/mL at 25 °C (lit.)
SMILES string
[Mn++].[O-]C([O-])=O
InChI
1S/CH2O3.Mn/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
XMWCXZJXESXBBY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- A primary electrode material in asymmetric supercapacitors for improving the charge storage capacity and overall performance of the supercapacitors.
- A precursor for synthesizing manganese oxide, which is used as a component in various electrochemical applications, including batteries and supercapacitors.
- A precursor material in the synthesis of oxygen vacancy-rich nitrogen-doped manganese carbonate (MnCO2@N) microspheres. These microspheres are then employed as cathode materials in aqueous zinc-ion batteries (ZIBs) to enhance their electrochemical performance.
- A potential electrocatalyst for the oxygen evolution reaction (OER) in water splitting applications.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Recently, layer-by-layer (LbL) assembly has emerged as a versatile, gentle and, simple method for immobilization of functional molecules in an easily controllable thin film morphology.1,2 In this short review, we introduce recent advances in functional systems fabricated by using the mild, yet adaptable LbL technique.
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service