377155
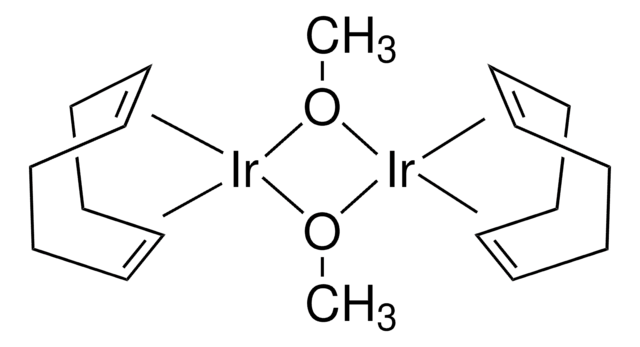
Chlorobis(cyclooctene)iridium(I)dimer
97%
Synonym(s):
[Ir(coe)2Cl]2, Di-μ-chlorotetrakis(cyclooctene)diiridium
About This Item
Recommended Products
Assay
97%
form
powder
reaction suitability
core: iridium
reagent type: catalyst
mp
160-165 °C (dec.) (lit.)
SMILES string
Cl[Ir].Cl[Ir].[CH]1[CH]CCCCCC1.[CH]2[CH]CCCCCC2.[CH]3[CH]CCCCCC3.[CH]4[CH]CCCCCC4
InChI
1S/4C8H14.2ClH.2Ir/c4*1-2-4-6-8-7-5-3-1;;;;/h4*1-2H,3-8H2;2*1H;;/q;;;;;;2*+1/p-2/b4*2-1-;;;;
InChI key
WBRREXQCZAFSKS-XFCUKONHSA-L
Related Categories
Application
- Isomerization-hydroboration reactions with nido-carboranyldiphosphine as stabilizing ligand
- Hydrogen peroxide oxidation of hydroxamic acids and their subsequent hetero Diels-Alder cycloaddition reactions
- Immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions
- Alkylation reactions
- Guerbet reaction
- Allylic amination reactions in a DNA-diene-iridium(I) hybrid system
- Asymmetric hydroamination reactions
- Isomerization-hydroboration reactions with nido-carboranyldiphosphine as stabilizing ligand.
- Hydrogen peroxide oxidation of hydroxamic acids and their subsequent hetero Diels-Alder cycloaddition reactions.
- Immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions.
- Alkylation reactions.
- Guerbet reaction.
- Allylic amination reactions using a DNA-diene-iridium(I) hybrid system.
- Asymmetric hydroamination reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Arylboronic acids and esters, vital tools in chemical transformations, find extensive use, particularly in the Suzuki-Miyaura cross-coupling reaction.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![[Ir(cod)(acac)] Umicore](/deepweb/assets/sigmaaldrich/product/structures/188/615/470bfca9-6b61-476a-9486-f7da61962e4c/640/470bfca9-6b61-476a-9486-f7da61962e4c.png)