338419
(R)-(+)-3-Chloro-1-phenyl-1-propanol
98%
Synonym(s):
α-(2-Chloroethyl)benzyl alcohol, (R)-(+)-α-(2-Chloroethyl)benzyl alcohol, (R)-(+)-3-Chloro-1-phenylpropanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
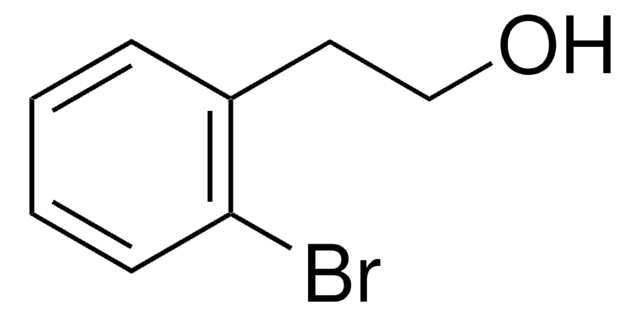
ClCH2CH2CH(C6H5)OH
CAS Number:
Molecular Weight:
170.64
Beilstein:
5250766
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
optical activity
[α]24/D +26°, c = 1 in chloroform
optical purity
ee: 99% (GLC)
mp
58-60 °C (lit.)
SMILES string
O[C@H](CCCl)c1ccccc1
InChI
1S/C9H11ClO/c10-7-6-9(11)8-4-2-1-3-5-8/h1-5,9,11H,6-7H2/t9-/m1/s1
InChI key
JZFUHAGLMZWKTF-SECBINFHSA-N
Application
(R)-(+)-3-Chloro-1-phenyl-1-propanol may be used as a building block in the synthesis of antidepressants such as (R)- and (S)-tomoxetine, fluoxetine and nisoxetine. It may also be used in the synthesis of biologically active 2-substituted chromans such as tephrowatsin E.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A stereocontrolled route to 2-substituted chromans.
Hodgetts KJ.
Tetrahedron Letters, 41(44), 8655-8659 (2000)
An efficient route to enantiomerically pure antidepressants: tomoxetine, nisoxetine and fluoxetine.
Schneider MP and Goergens U.
Tetrahedron Asymmetry, 3(4), 525-528 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service