All Photos(1)
About This Item
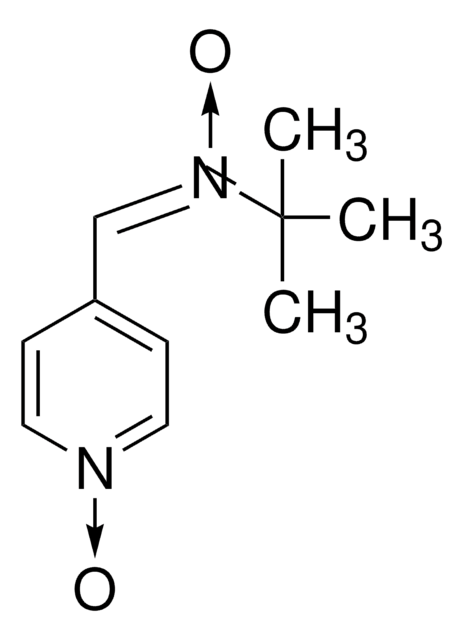
Empirical Formula (Hill Notation):
C8H15NO
CAS Number:
Molecular Weight:
141.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
73 °C/1 mmHg (lit.)
mp
58-61 °C (lit.)
storage temp.
−20°C
SMILES string
CC1(C)CC(C)(C)[N+]([O-])=C1
InChI
1S/C8H15NO/c1-7(2)5-8(3,4)9(10)6-7/h6H,5H2,1-4H3
InChI key
GUQARRULARNYQZ-UHFFFAOYSA-N
General description
The ESR spectrum of 3,3,5,5-tetramethyl-1-pyrroline N-oxide was studied.
Application
3,3,5,5-Tetramethyl-1-pyrroline N-oxide was used as a spin traping reagent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G M Rosen et al.
Journal of medicinal chemistry, 31(2), 428-432 (1988-02-01)
Two nitrones, 3,3-diethyl-5,5-dimethylpyrroline 1-oxide (DEDMPO) and 3,3,5,5-tetramethylpyrroline 1-oxide (M4PO), were synthesized by the zinc/ammonium chloride reduction of appropiately substituted gamma-nitrocarbonyl compounds, followed by addition of methylmagnesium bromide to the resulting intermediate nitrones. The lipophilicities of these nitrones were estimated by
S Unchern et al.
Neurochemical research, 23(1), 97-102 (1998-03-03)
We compared neurotoxicity of piperine and low K+ on cultured cerebellar granule neurons. As considered from lactate dehydrogenase release and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide reduction, both piperine and shifting from high K+ (25 mM) to low K+ (5.4 mM) were
M J Davies et al.
The Biochemical journal, 240(3), 789-795 (1986-12-15)
Spin trapping using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) has been used to detect and distinguish between the carbon-centred, alkoxyl, and peroxyl radicals produced during the photolytic decomposition of hydroperoxides. Photolysis of tert-butyl and cumene hydroperoxides, and peroxidized fatty acids, in toluene, with
Shing-Jong Huang et al.
Solid state nuclear magnetic resonance, 29(4), 272-277 (2005-11-09)
We show that a Gaussian-shaped pulse can be used to excite selected 1H signals in hydroxyapatite, monetite and H-Y zeolite loaded with trimethylphosphine oxide (TMPO). This selective excitation method can be incorporated into Lee-Goldburg (LG) cross-polarization to obtain useful spectral
E G Janzen et al.
Journal of biochemical and biophysical methods, 30(4), 239-247 (1995-11-01)
To obtain the strongest possible free radical spin adduct signal using the electron paramagnetic resonance spectroscopy-spin trapping technique, it is desirable to load an animal with the highest dose of spin trap possible. One hundred and twenty six male Sprague-Dawley
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service