178098
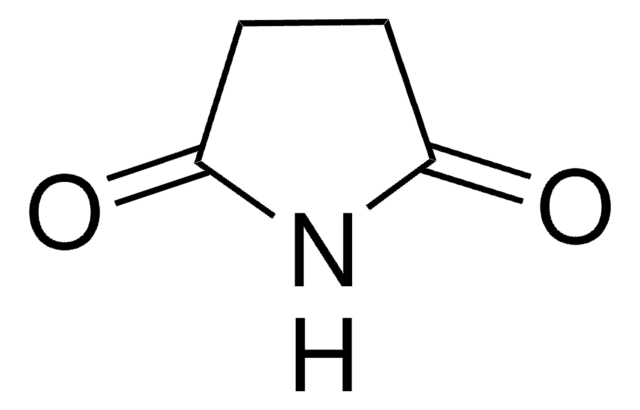
Glutarimide
98%
Synonym(s):
2,6-Piperidinedione, NSC 58190
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
155-157 °C (lit.)
SMILES string
O=C1CCCC(=O)N1
InChI
1S/C5H7NO2/c7-4-2-1-3-5(8)6-4/h1-3H2,(H,6,7,8)
InChI key
KNCYXPMJDCCGSJ-UHFFFAOYSA-N
General description
A glutarimide antibiotic, 9-methylstreptimidone, shows antiviral, antitumor and antifungal activities.
Application
Reactant for:
Thionations
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture
Synthesis of β-adrenoceptor ligands
Enantioselective synthesis of securinega alkaloids
Intramolecular amidocyclopropanation reactions
Synthesis of alpha-fluoro-alpha amino amides
Thionations
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture
Synthesis of β-adrenoceptor ligands
Enantioselective synthesis of securinega alkaloids
Intramolecular amidocyclopropanation reactions
Synthesis of alpha-fluoro-alpha amino amides
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jianhua Ju et al.
Journal of the American Chemical Society, 131(4), 1370-1371 (2009-01-10)
Migrastatin (1), iso-migrastatin (5) and lactimidomycin (7) are all glutarimide-containing polyketides known for their unique structures and cytotoxic activities against human cancer cell lines. Migrastatin, a strong inhibitor of tumor cell migration, has been an important lead in the development
Jianhua Ju et al.
Bioorganic & medicinal chemistry letters, 18(22), 5951-5954 (2008-08-08)
Lactimidomycin (LTM, 1), iso-migrastatin (iso-MGS, 2) and migrastatin (MGS, 3) are macrolide antitumor antibiotics differing in macrolide ring size but all bearing a glutarimide side chain. To further develop these natural products and related analogs as drug candidates we have
Jian Wei et al.
Nature nanotechnology, 3(8), 496-500 (2008-08-08)
The submillimetre or terahertz region of the electromagnetic spectrum contains approximately half of the total luminosity of the Universe and 98% of all the photons emitted since the Big Bang. This radiation is strongly absorbed in the Earth's atmosphere, so
Yuan-Ping Ruan et al.
Chirality, 17(9), 595-599 (2005-10-04)
An analytical HPLC method using CHIREX (S)-LEU/(S)-alpha-NEA column was developed for the determination of the enantiomeric excesses of N-protected (S)-3-hydroxyglutarimides. Using this method, detailed studies on the base-promoted ring-expansion reaction of the amidolactones, derived from l-glutamic acid, were undertaken.
Bo Wang et al.
Organic letters, 15(6), 1278-1281 (2013-02-27)
9-Methylstreptimidone is a glutarimide antibiotic showing antiviral, antifungal, and antitumor activities. Genome scanning, bioinformatics analysis, and gene inactivation experiments reveal a gene cluster responsible for the biosynthesis of 9-methylstreptimidone in Streptomyces himastatinicus. The unveiled machinery features both acyltransferase- and thioesterase-less
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service