All Photos(1)
About This Item
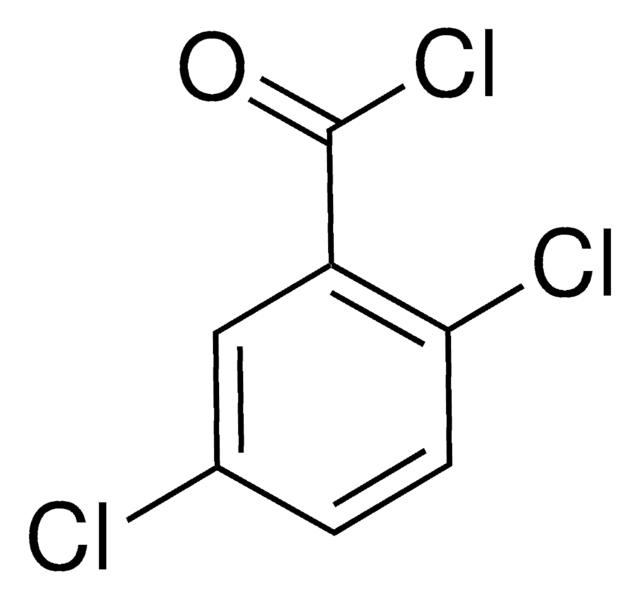
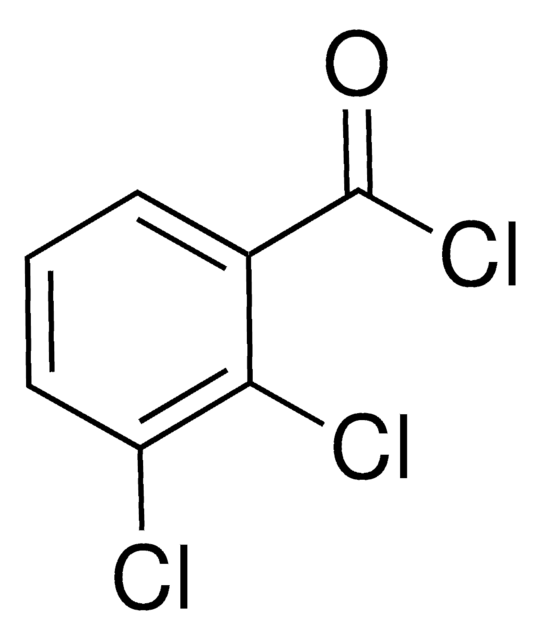
Linear Formula:
Cl2C6H3COCl
CAS Number:
Molecular Weight:
209.46
Beilstein:
607485
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39050528
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
242 °C (lit.)
mp
30-33 °C (lit.)
SMILES string
ClC(=O)c1ccc(Cl)c(Cl)c1
InChI
1S/C7H3Cl3O/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3H
InChI key
VTXNOVCTHUBABW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,4-Dichlorobenzoyl chloride was used in the synthesis of 3,4-dichlorophenylacetic acid by Arndt-Eistert method. It is prepared by refluxing 3,4-dichlorobenzoic acid with thionyl chloride.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
287.6 °F - closed cup
Flash Point(C)
142 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Inhibitors of the Hill reaction.
N E Good
Plant physiology, 36(6), 788-803 (1961-11-01)
Hydroxyindole-O-methyltransferase. 3. Influ- ence of the phenyl moiety on the inhibitory activities of some N-acyltryptamines.
B T Ho et al.
Journal of pharmaceutical sciences, 58(5), 563-566 (1969-05-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service