913294
keYPhos™
Umicore
Synonym(s):
CyYPhos(Me)PCy2, Tricyclohexyl(1-(dicyclohexyl-phosphanyl)ethylidene)-phosphane
About This Item
Recommended Products
product name
keYPhos™,
form
powder
Quality Level
reaction suitability
reagent type: ligand
mp
167-169 °C
functional group
phosphine
General description
Application
Learn more about ylide-functionalized phosphines (YPhos)
Features and Benefits
Legal Information
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Electron-rich ylide-substituted phosphine ligands allow for palladium catalyzed coupling reactions at mild reaction conditions. These YPhos ligands enable the conversion of aryl chlorides with short reaction times.
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service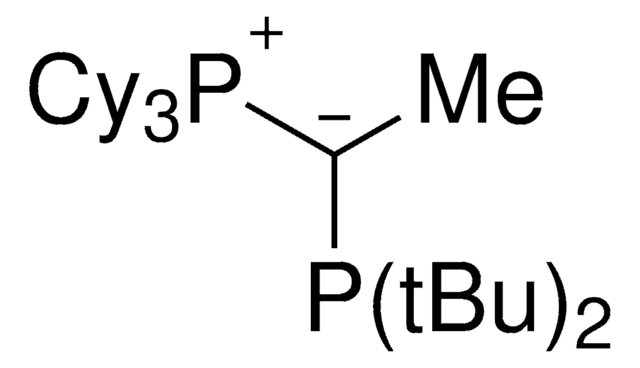
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)






![Dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/351/904/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3/640/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3.png)