All Photos(1)
About This Item
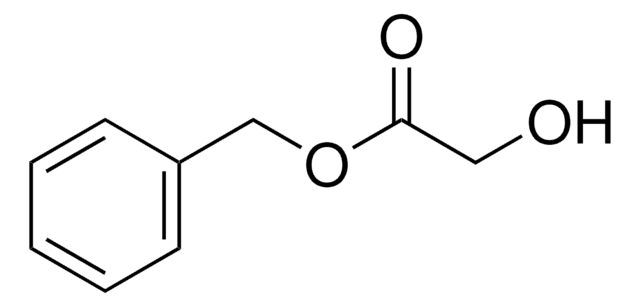
Linear Formula:
C6H5CH2OCH2CO2H
CAS Number:
Molecular Weight:
166.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.526 (lit.)
bp
137-139 °C/0.6 mmHg (lit.)
density
1.162 g/mL at 25 °C (lit.)
functional group
carboxylic acid
ether
phenyl
SMILES string
OC(=O)COCc1ccccc1
InChI
1S/C9H10O3/c10-9(11)7-12-6-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,10,11)
InChI key
GRZHHTYDZVRPIC-UHFFFAOYSA-N
General description
In vitro activities of phenoxy and benzyloxyacetic acid derivatives as antisickling agents has been investigated by the application of quantitative structure activity relationship (QSAR) of Hansch-type.
Application
Benzyloxyacetic acid may be used for the following syntheses:
- mixed benzyloxyacetic pivalic anhydride
- chiral glycolates, 1,2:4,5-di-O-isopropylidene-β-D-fructopyranos-3-yl benzyloxyacetate, via esterification
- tetraaqua(1,10-phenanthroline-[κ]2N,N′)magnesium(II) bis[(2,4-dichlorophenyl)acetate]
- as ligand in the preparation of diaquabis(benzyloxyacetato)copper(II) complex
Other Notes
may contain chloroacetic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A highly effective asymmetric synthesis of a-hydroxy acids by alkylation of chiral n-(benzyloxyacetyl)-trans-2, 5-bis (methoxymethoxymethyl) pyrrolidine.
Enomoto M, et al.
Tetrahedron Letters, 26(10), 1343-1344 (1985)
Xiao-Min Hao et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 8), m1052-m1052 (2008-01-01)
In the mononuclear title complex, [Mg(C(12)H(8)N(2))(H(2)O)(4)](C(8)H(5)Cl(2)O(2))(2), each Mg(II) ion is hexa-coordinated by two N atoms from a 1,10-phenanthroline ligand [Mg-N = 2.233 (2) Å] and four water mol-ecules [Mg-OW = 2.033 (2) and 2.043 (1) Å] in a distorted octa-hedral geometry. A twofold rotation axis
M A Mahran
Bollettino chimico farmaceutico, 139(2), 73-80 (2000-08-02)
Quantitative structure activity relationship of Hansch-type has been applied to develop correlation between the calculated physicochemical properties and the in vitro activities of phenoxy and benzyloxyacetic acid derivatives as antisickling agents. The antisickling effect of these compounds was first reported
Diaquabis (benzyloxyacetato) copper (II).
Sun S-L, et al.
Acta Crystallographica Section E, Structure Reports Online, 64(5), 1052-1052 (2008)
An inexpensive carbohydrate derivative used as a chiral auxiliary in the synthesis of a-hydroxy carboxylic acids.
Yu H, et al.
Tetrahedron, 58(38), 7663-7669 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)