SMB00311
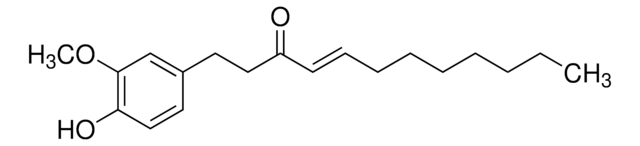
Shogaol
≥90% (HPLC)
Sinónimos:
[6]-Shogaol, (6)-Shogaol, 1-(4-Hydroxy-3-methoxyphenyl)-4-decen-3-one
About This Item
Productos recomendados
Ensayo
≥90% (HPLC)
Formulario
liquid
aplicaciones
metabolomics
vitamins, nutraceuticals, and natural products
temp. de almacenamiento
2-8°C
cadena SMILES
CCCCC\C=C\C(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C17H24O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h7-8,10,12-13,19H,3-6,9,11H2,1-2H3/b8-7+
Clave InChI
OQWKEEOHDMUXEO-BQYQJAHWSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![[6]-Shogaol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/378/737/6e6bd2f9-0152-45e1-8006-344ca92937be/640/6e6bd2f9-0152-45e1-8006-344ca92937be.png)
![[6]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/259/444/6877889c-1cc0-47f5-b807-f847deadec1d/640/6877889c-1cc0-47f5-b807-f847deadec1d.png)


![[10]-Gingerol ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)


![[6]-Gingerol, Zingiber officinale An antitumor, apoptosis-inducing compound of the ginger family that blocks EGF-induced cell transformation by inhibiting the activation of Activator Protein-1 (AP-1).](/deepweb/assets/sigmaaldrich/product/images/140/919/0846df46-0b67-4c28-b99d-87e177be65b2/640/0846df46-0b67-4c28-b99d-87e177be65b2.jpg)