S8559
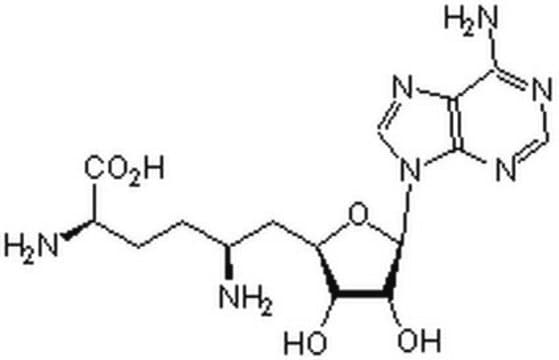
Sinefungin
95% (HPLC), powder, methylation of bases in DNA & RNA inhibitor
Sinónimos:
5′-Deoxy-5′-(1,4-diamino-4-carboxybutyl)adenosine, A-9145, Adenosylornithine, Antibiotic 32232RP
About This Item
Productos recomendados
product name
Sinefungin, 95% (HPLC), powder
Quality Level
assay
95% (HPLC)
form
powder
color
white to yellow
solubility
H2O: complete 20 mg/ml, clear, colorless to light yellow
H2O: soluble
antibiotic activity spectrum
neoplastics
mode of action
DNA synthesis | interferes
enzyme | inhibits
storage temp.
2-8°C
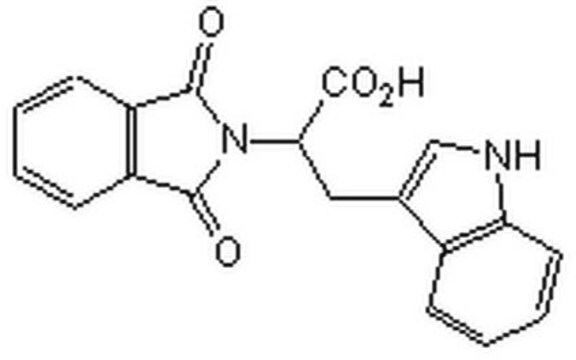
SMILES string
N[C@@H](CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C15H23N7O5/c16-6(1-2-7(17)15(25)26)3-8-10(23)11(24)14(27-8)22-5-21-9-12(18)19-4-20-13(9)22/h4-8,10-11,14,23-24H,1-3,16-17H2,(H,25,26)(H2,18,19,20)/t6-,7-,8+,10+,11+,14+/m0/s1
InChI key
LMXOHSDXUQEUSF-YECHIGJVSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Biochem/physiol Actions
Methylation inhibition by sinefugin is often accompanied by an altered rate of cytosine deamination that is coupled to transition mutation in the DNA. Sinefugin inhibits Epstein-Barr viral activity and this inhibition is related to the change in DNA methylation and gene expression. It can cause a rate change in several restriction DNA endonuclease activities, including Mme I, which is not connected to the inhibition of the methytransferase activity.
Features and Benefits
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico