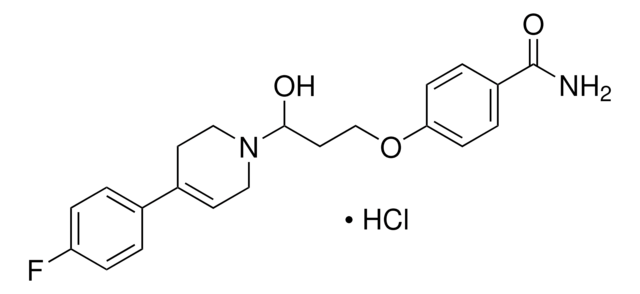
I119
Indatraline hydrochloride
solid
Sinónimos:
(±)-trans-3-(3,4-Dichlorophenyl)-N-methyl-1-indanamine hydrochloride, Lu 19-005
About This Item
Productos recomendados
Formulario
solid
Nivel de calidad
color
white
solubilidad
H2O: 2 mg/mL
temp. de almacenamiento
2-8°C
cadena SMILES
Cl.CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c3ccccc13
InChI
1S/C16H15Cl2N.ClH/c1-19-16-9-13(11-4-2-3-5-12(11)16)10-6-7-14(17)15(18)8-10;/h2-8,13,16,19H,9H2,1H3;1H/t13-,16+;/m0./s1
Clave InChI
QICQDZXGZOVTEF-MELYUZJYSA-N
Información sobre el gen
human ... DRD1(1812) , DRD2(1813) , DRD3(1814) , DRD4(1815) , DRD5(1816) , HTR1A(3350) , HTR1B(3351) , HTR1D(3352) , HTR1E(3354) , HTR1F(3355) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358) , HTR3A(3359) , HTR3B(9177) , HTR3C(170572) , HTR3D(200909) , HTR3E(285242) , HTR4(3360) , HTR5A(3361) , HTR5B(645694) , HTR6(3362) , HTR7(3363)
Aplicación
- as a competitive inhibitor of 3H-dopamine ([3H]DA) to study its effects on trans-activator of transcription (Tat) protein on cocaine-induced inhibition of uptake of [3H]DA
- as a dopamine transport blocker to study its effects on trace amine-associated receptor 1 (TAAR1)-transfected mice cells
- as a nonselective monoamine transport inhibitor to study its anti-angiogenic activities in glioblastoma multiforme (GBM)
Acciones bioquímicas o fisiológicas
Características y beneficios
Información legal
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Aquatic Acute 1 - Aquatic Chronic 1
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico