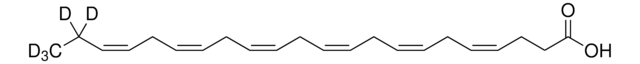
D2534
cis-4,7,10,13,16,19-Docosahexaenoic acid
≥98%
Sinónimos:
DHA
About This Item
Productos recomendados
biological source
algae
Quality Level
assay
≥98%
form
liquid
functional group
carboxylic acid
lipid type
omega FAs
shipped in
dry ice
storage temp.
−20°C
SMILES string
CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(O)=O
InChI
1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-
InChI key
MBMBGCFOFBJSGT-KUBAVDMBSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- as a component in Dulbecco′s modified Eagle medium (DMEM) for culturing cells to perform docosahexaenoic acid (DHA) treatment
- to study its impact on human induced pluripotent stem cell (iPSC)-derived neuronal at a molecular and cellular level
- in MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay to study its cytotoxic effects on three human hepatocellular carcinoma (HCC) cell lines (HepG2, Hep3B, Huh7)
Biochem/physiol Actions
Packaging
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
143.6 °F - closed cup
flash_point_c
62 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Lipid Induced Insulin Resistance
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico