B3131
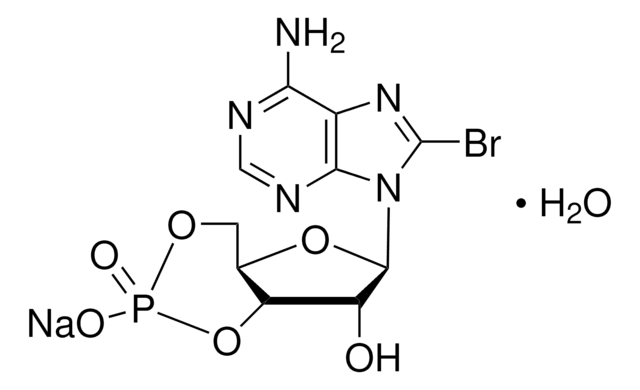
8-Bromoadenosine 5′-monophosphate
≥98%
Sinónimos:
8-Br-AMP
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H13BrN5O7P
Número de CAS:
Peso molecular:
426.12
Número MDL:
Código UNSPSC:
41106305
ID de la sustancia en PubChem:
NACRES:
NA.51
Productos recomendados
origen biológico
synthetic (organic)
Nivel de calidad
Ensayo
≥98%
Formulario
powder
solubilidad
water: 100 mg/mL, clear, colorless
temp. de almacenamiento
−20°C
cadena SMILES
Nc1ncnc2n(C3OC(COP(O)(O)=O)C(O)C3O)c(Br)nc12
InChI
1S/C10H13BrN5O7P/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,13,14)(H2,19,20,21)
Clave InChI
DNPIJKNXFSPNNY-UHFFFAOYSA-N
Categorías relacionadas
Aplicación
8-Bromoadenosine 5′′-monophosphate (8-Br-AMP) is an analogue of 5′-AMP useful for receptor mapping studies; as a starting structure for 8-modified 5′-AMP derivatives and for synthesis of poly-8-bromoriboadenylic acid.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
J O Folayan et al.
Journal of biochemistry, 96(4), 1297-1301 (1984-10-01)
Poly-8-bromoriboadenylic acid was synthesized by the bromination of adenosine-5'-monophosphate to yield 8-bromoadenosine-5'-monophosphate which on conversion to the 5'-diphosphate form was polymerized by polynucleotide phosphorylase (PNPase). The polymer formed a 1:1 hybrid with polyribouridylic acid and the hybrid was found to
Rongkuan Hu et al.
PloS one, 8(8), e73527-e73527 (2013-08-24)
This study is the first to demonstrate that shizukaol D, a natural compound isolated from Chloranthusjaponicus, can activate AMP- activated protein kinase (AMPK), a key sensor and regulator of intracellular energy metabolism, leading to a decrease in triglyceride and cholesterol
K Lesiak et al.
Biochemical and biophysical research communications, 126(2), 917-921 (1985-01-31)
When added to extracts of mouse L cells containing ATP and an energy regenerating system, the 5'-diphosphate of 2-5A core, pp5'A2'p5'A2'p5'A, as well as a bromoadenylate analog, pp5' (br8A)2'p5'(br8A)2'p5'(br8A), can be phosphorylated to the corresponding 5'-triphosphate, ppp5'A2'p5'A2'p5'A and ppp5'(br8A)2'p5'(br8A)2'p5(br8A), respectively.
Sophia E Airhart et al.
PloS one, 12(12), e0186459-e0186459 (2017-12-07)
The co-primary objectives of this study were to determine the human pharmacokinetics (PK) of oral NR and the effect of NR on whole blood nicotinamide adenine dinucleotide (NAD+) levels. Though mitochondrial dysfunction plays a critical role in the development and
Vikram J Tallapragada et al.
The Journal of pharmacology and experimental therapeutics, 356(2), 424-433 (2015-11-19)
The ventrolateral medulla contains presympathetic and vagal preganglionic neurons that control vasomotor and cardiac vagal tone, respectively. G protein-coupled receptors influence the activity of these neurons. Gα s activates adenylyl cyclases, which drive cyclic adenosine monophosphate (cAMP)-dependent targets: protein kinase
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico