PHR1039
Erythromycin
Pharmaceutical Secondary Standard; Certified Reference Material
About This Item
Productos recomendados
grade
certified reference material
pharmaceutical secondary standard
Quality Level
agency
traceable to BP 794
traceable to Ph. Eur. E1305000
traceable to USP 1242000
API family
erythromycin
CofA
current certificate can be downloaded
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
-10 to -25°C
SMILES string
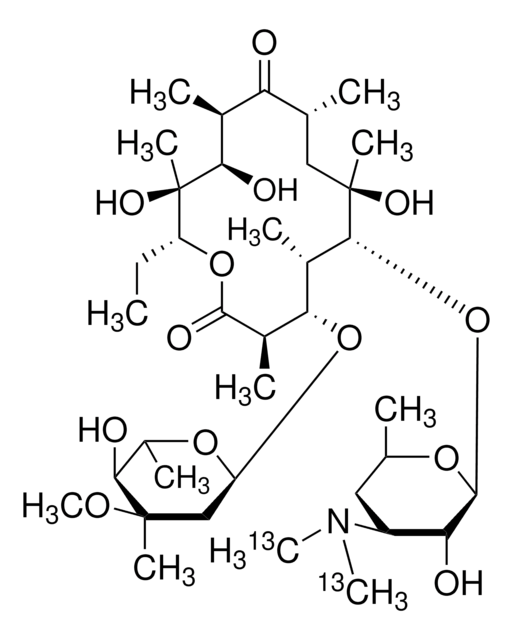
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
InChI key
ULGZDMOVFRHVEP-RWJQBGPGSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Erythromycin is a complex macrolide antibiotic drug that exhibits a bacteriostatic activity. It is generally employed in human and veterinary medicines owing to its potential activity against gram positive and a few gram negative strains.
Application
Biochem/physiol Actions
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Caution
Preparation Note
Footnote
Legal Information
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available. This product was designed, produced and verified for accuracy and stability in accordance with ISO/IEC 17025:2005 (AClass Cert AT-1467), ISO GUIDES 34:2009 (AClass Cert AR-1470).
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Comparative analysis of Supel™ BioSPME 96-Pin device with a rapid equilibrium dialysis technique for accuracy of measured values, sample cleanliness, and workflow time in drug protein binding.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico





![(1S,6R)-6-HYDROXY-5-METHYLENE-1-[(1S)-1,2,3-TRIHYDROXY-2-METHYLPROPYL]-2-OXA-7,9-DIAZABICYCLO[4.2.2]DECANE-8,10-DIONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/271/983/0a99c1ad-6200-4f41-b31f-bd929e66599d/640/0a99c1ad-6200-4f41-b31f-bd929e66599d.png)