H0920000
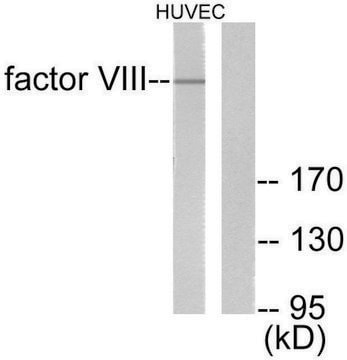
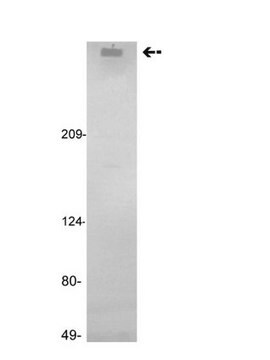
Human coagulation factor VIII concentrate
European Pharmacopoeia (EP) Reference Standard
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
grade
pharmaceutical primary standard
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Human coagulation factor VIII concentrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Innovations in coagulation: improved options for treatment of hemophilia A and B.
Ingrid Pabinger-Fasching et al.
Thrombosis research, 131 Suppl 2, S1-S1 (2013-03-30)
Puneet Gaitonde et al.
The Journal of biological chemistry, 288(24), 17051-17056 (2013-05-08)
Administration of recombinant factor VIII (FVIII), an important co-factor in blood clotting cascade, elicits unwanted anti-FVIII antibodies in hemophilia A (HA) patients. Previously, FVIII associated with phosphatidylserine (PS) showed significant reduction in the anti-FVIII antibody response in HA mice. The
Samantha C Gouw et al.
Blood, 121(20), 4046-4055 (2013-04-05)
The objective of this study was to examine the association of the intensity of treatment, ranging from high-dose intensive factor VIII (FVIII) treatment to prophylactic treatment, with the inhibitor incidence among previously untreated patients with severe hemophilia A. This cohort
F8 gene and phenotype: single player in a team?
Anna Pavlova
Blood, 121(19), 3784-3785 (2013-05-11)
Factor VIII products and inhibitors in severe hemophilia A.
Alfonso Iorio et al.
The New England journal of medicine, 368(15), 1456-1456 (2013-04-12)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico