402869
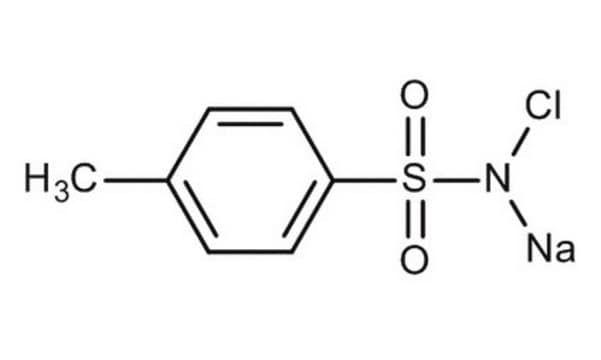
Chloramine T trihydrate
ACS reagent, 98%
Sinónimos:
N-Chloro-p-toluenesulfonamide sodium salt
About This Item
Productos recomendados
grade
ACS reagent
Quality Level
assay
98%
98.0-103.0% (ACS specification)
form
powder
reaction suitability
reagent type: oxidant
impurities
<1.5% insolubles (alcohol)
pH
8.0-10.0 (25 °C, 5%)
mp
167-170 °C (lit.)
solubility
H2O: passes test
suitability
passes test for determination of bromide
SMILES string
O.O.O.Cc1ccc(cc1)S(=O)(=O)N([Na])Cl
InChI
1S/C7H7ClNO2S.Na.3H2O/c1-6-2-4-7(5-3-6)12(10,11)9-8;;;;/h2-5H,1H3;;3*1H2/q-1;+1;;;
InChI key
NZYOAGBNMCVQIV-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
It may also be used in the synthesis of the following compounds:
- Nitrile imines by the oxidative dehydrogenation of N-(nitrobenzyl)-imidazole aldehyde hydrazine.
- Mono-N-tosylated-1,2-diamines.
- 3,5-Disubstituted isoxazoles.
- Fused 3,6-disubstituted triazolothiadiazoles by the oxidative cyclization of N-heteroaryl-substituted hydrazones.
Packaging
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B
supp_hazards
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 2
flash_point_f
377.6 °F - closed cup
flash_point_c
192 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico