00878
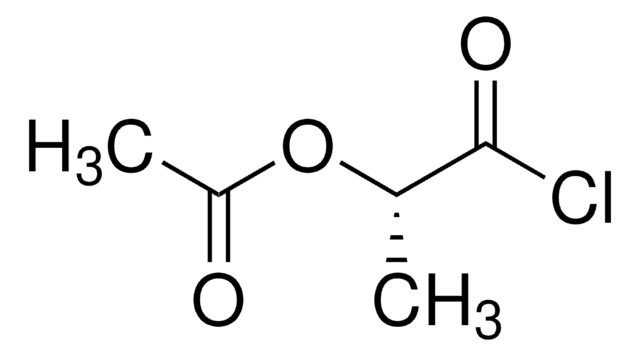
(±)-2-Acetoxypropionic acid
≥97.0% (GC)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3COOCH(CH3)COOH
Número de CAS:
Peso molecular:
132.11
Beilstein/REAXYS Number:
1722938
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥97.0% (GC)
refractive index
n20/D 1.423
density
1.176 g/mL at 20 °C (lit.)
functional group
carboxylic acid
ester
SMILES string
CC(OC(C)=O)C(O)=O
InChI
1S/C5H8O4/c1-3(5(7)8)9-4(2)6/h3H,1-2H3,(H,7,8)
InChI key
WTLNOANVTIKPEE-UHFFFAOYSA-N
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Michael Vinogradov et al.
Analytical biochemistry, 342(1), 126-133 (2005-06-17)
Acetohydroxy acid synthase (AHAS) and related enzymes catalyze the production of chiral compounds [(S)-acetolactate, (S)-acetohydroxybutyrate, or (R)-phenylacetylcarbinol] from achiral substrates (pyruvate, 2-ketobutyrate, or benzaldehyde). The common methods for the determination of AHAS activity have shortcomings. The colorimetric method for detection
Ahmet Baykal et al.
Bioorganic chemistry, 34(6), 380-393 (2006-11-07)
In addition to the decarboxylation of 2-oxo acids, thiamin diphosphate (ThDP)-dependent decarboxylases/dehydrogenases can also carry out so-called carboligation reactions, where the central ThDP-bound enamine intermediate reacts with electrophilic substrates. For example, the enzyme yeast pyruvate decarboxylase (YPDC, from Saccharomyces cerevisiae)
N Goupil et al.
Applied and environmental microbiology, 62(7), 2636-2640 (1996-07-01)
Diacetyl is a by-product of pyruvate metabolism in Lactococcus lactis, where pyruvate is first converted to alpha-acetolactate, which is slowly decarboxylated to diacetyl in the presence of oxygen. L. lactis usually converts alpha-acetolactate to acetoin enzymatically, by alpha-acetolactate decarboxylase encoded
Gonzalo Jaña et al.
Proteins, 78(7), 1774-1788 (2010-03-13)
Acetohydroxyacid synthase (AHAS) is a thiamin diphosphate dependent enzyme that catalyses the decarboxylation of pyruvate to yield the hydroxyethyl-thiamin diphosphate (ThDP) anion/enamine intermediate (HEThDP(-)). This intermediate reacts with a second ketoacid to form acetolactate or acetohydroxybutyrate as products. Whereas the
C Monnet et al.
Letters in applied microbiology, 36(6), 399-405 (2003-05-20)
To demonstrate the presence of an active alpha-acetolactate decarboxylase in Streptococcus thermophilus and to investigate its physiological function. Streptococcus thermophilus CNRZ385 contains a gene encoding an alpha-acetolactate decarboxylase. Comparison of the production of alpha-acetolactate and its decarboxylation products, by the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico