8.09622
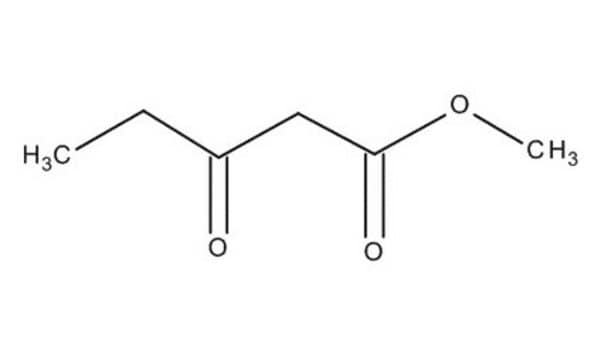
Ethyl acetoacetate
for synthesis
Sinónimos:
Ethyl acetoacetate, EAA, Acetoacetic acid ethyl ester
About This Item
Productos recomendados
vapor pressure
0.26 hPa ( 20 °C)
Quality Level
assay
≥98.0% (GC)
form
liquid
autoignition temp.
350 °C
potency
3980 mg/kg LD50, oral (Rat)
>5000 mg/kg LD50, skin (Rabbit)
expl. lim.
1.0-54 % (v/v)
pH
4.0 (20 °C, 110 g/L in H2O)
mp
-53.3 °C
transition temp
flash point 73.5 °C
solubility
130.3 g/L
density
1.03 g/cm3 at 20 °C
storage temp.
2-30°C
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
InChI key
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Application
- 7-hydroxycoumarin derivatives via Pechmann condensation reaction with 1,3-dihydroxybenzene in the presence of acid catalysts.
- 2,6-disubstituted piperidine alkaloid, (−)-pinidinone via stereoselective α-aminoallylation followed by Grubbs′ olefin cross-metathesis reaction.
- Michael addition products via Michael addition reaction with chalcones and azachalcones in the presence of a base catalyst.
- α, β-unsaturated carbonyl compounds via Knoevenagel condensation reaction with glyceraldehyde acetonide in the presence of a base catalyst.
It can be also utilized as a reactant in the transesterification and asymmetric hydrogenation reactions to produce valuable products.
Analysis Note
Density (d 20 °C/ 4 °C): 1.028 - 1.030
Identity (IR): passes test
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
164.3 °F - closed cup
flash_point_c
73.5 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico