W309311
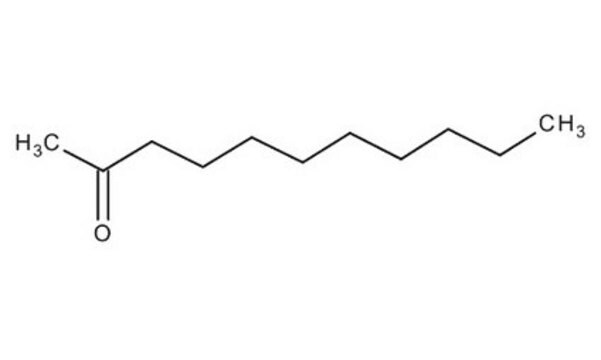
2-Undecanone
natural, FCC, FG
Sinónimos:
Methyl nonyl ketone
About This Item
Productos recomendados
grade
FG
Halal
Kosher
natural
Quality Level
agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
vapor density
5.9 (vs air)
vapor pressure
<1 mmHg ( 20 °C)
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.43 (lit.)
bp
231-232 °C (lit.)
mp
11-13 °C (lit.)
density
0.825 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
greener alternative category
organoleptic
fatty; waxy; fruity
SMILES string
CCCCCCCCCC(C)=O
InChI
1S/C11H22O/c1-3-4-5-6-7-8-9-10-11(2)12/h3-10H2,1-2H3
InChI key
KYWIYKKSMDLRDC-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Larvicididal Activity of Natural Repellents Against the Dengue Vector, Aedes aegypti: This paper details the use of natural compounds like 2-Undecanone as effective larvicidal agents against vectors of significant human diseases, showcasing their role in developing non-toxic, eco-friendly insect control solutions (Zhang et al., 2020).
- Control of Filth Flies, Cochliomyia macellaria (Diptera: Calliphoridae), Musca domestica (Diptera: Muscidae), and Sarcophaga bullata (Diptera: Sarcophagidae), Using Novel Plant-Derived Methyl Ketones: Investigates the efficacy of 2-Undecanone in managing common pest flies, underlining its potential as a biopesticide and organic solvent in public health and safety applications (Deguenon et al., 2019).
Biochem/physiol Actions
signalword
Warning
hcodes
pcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
192.2 °F - closed cup
flash_point_c
89 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico