G7208
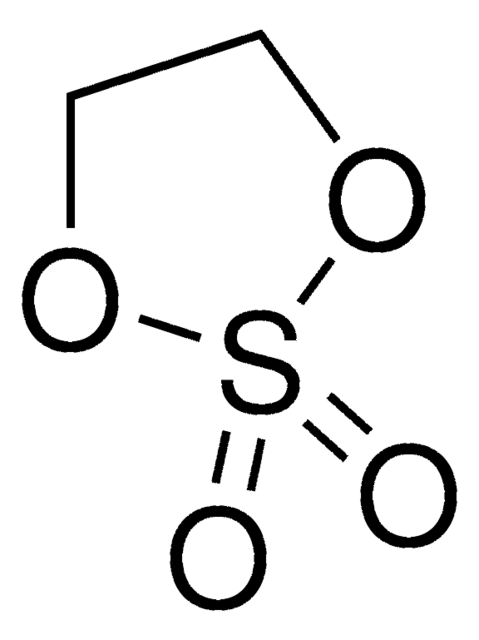
Ethylene sulfite
98%
Sinónimos:
1,3,2-Dioxathiolan-2-oxide, Cyclic ethylene sulfite, ES, Glycol sulfite
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C2H4O3S
Número de CAS:
Peso molecular:
108.12
Beilstein/REAXYS Number:
1237109
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.445 (lit.)
bp
159.1 °C (lit.)
density
1.426 g/mL at 25 °C (lit.)
SMILES string
O=S1OCCO1
InChI
1S/C2H4O3S/c3-6-4-1-2-5-6/h1-2H2
InChI key
WDXYVJKNSMILOQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
197.1 °F
flash_point_c
91.7 °C
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Joseph P O'Shea et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 207-216 (2015-07-29)
Novel formulations that overcome the solubility limitations of poorly water soluble drugs (PWSD) are becoming ever more critical to a drug development process inundated with these compounds. There is a clear need for developing bio-enabling formulation approaches to improve oral
Dharmendra K Yadav et al.
AAPS PharmSciTech, 16(4), 855-864 (2015-01-15)
The objective of this study was to develop novel docetaxel phospholipid nanoparticles (NDPNs) for intravenous administration. Modified solvent diffusion-evaporation method was adopted in the NDPN preparation. Central composite design (CCD) was employed in the optimization of the critical formulation factor
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(6), 1734-1746 (2014-04-18)
The current study determined the extent to which the desorption of lipid-based formulations (LBFs) from a mesoporous magnesium aluminometasilicate (Neusilin®-US2) carrier is governed by drug properties, LBF composition, and LBF-to-adsorbent ratio. A secondary objective was to evaluate the impact of
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(8), 2441-2455 (2014-07-06)
The Lipid Formulation Classification System Consortium looks to develop standardized in vitro tests and to generate much-needed performance criteria for lipid-based formulations (LBFs). This article highlights the value of performing a second, more stressful digestion test to identify LBFs near
Mette U Anby et al.
Molecular pharmaceutics, 11(11), 4069-4083 (2014-09-30)
The impact of gastrointestinal (GI) processing and first pass metabolism on danazol oral bioavailability (BA) was evaluated after administration of self-emulsifying drug delivery systems (SEDDS) in the rat. Danazol absolute BA was determined following oral and intraduodenal (ID) administration of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico