E35400
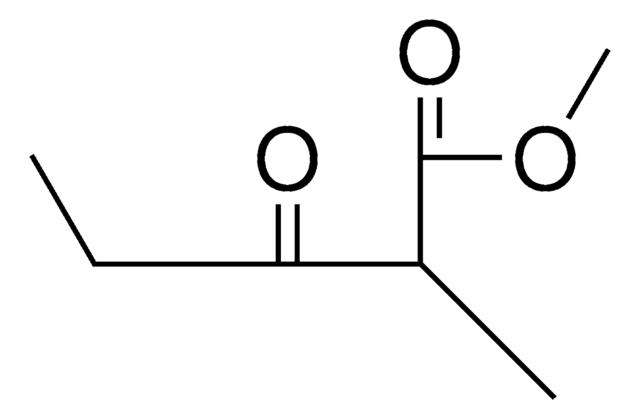
Ethyl 2-methylacetoacetate
90%
Sinónimos:
Ethyl 2-methyl-3-oxobutanoate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3COCH(CH3)CO2C2H5
Número de CAS:
Peso molecular:
144.17
Beilstein/REAXYS Number:
1071742
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
90%
form
liquid
refractive index
n20/D 1.418 (lit.)
bp
187 °C (lit.)
density
1.019 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(C)C(C)=O
InChI
1S/C7H12O3/c1-4-10-7(9)5(2)6(3)8/h5H,4H2,1-3H3
InChI key
FNENWZWNOPCZGK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Ethyl 2-methylacetoacetate is used as a substrate in the rhenium-catalyzed synthesis of multisubstituted aromatic compounds.
- It can be employed in the synthesis of coumarin derivatives via Pechmann condensation.
- It undergoes dehydration to yield conjugated alkynyl and allenyl esters.
- It is also used in the total synthesis of chlorotonil A, yangjinhualine A, (+)- and (−)-saudin.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
145.4 °F - closed cup
flash_point_c
63 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Concise, regiocontrolled synthesis of yangjinhualine A.
Boukouvalas J and McCann L C
Tetrahedron Letters, 52(11), 1202-1204 (2011)
An enantioselective total synthesis of (+)-and (−)-saudin. Determination of the absolute configuration.
Boeckman R K, et al.
Journal of the American Chemical Society, 124(2), 190-191 (2002)
Michal Plž et al.
Molecules (Basel, Switzerland), 25(18) (2020-09-24)
The co-immobilization of ketoreductase (KRED) and glucose dehydrogenase (GDH) on highly cross-linked agarose (sepharose) was studied. Immobilization of these two enzymes was performed via affinity interaction between His-tagged enzymes (six histidine residues on the N-terminus of the protein) and agarose
T Kuramoto et al.
Bioscience, biotechnology, and biochemistry, 63(3), 598-601 (1999-05-05)
Chlorella pyrenoidosa Chick reduced ethyl 2-methyl 3-oxobutanoate to the corresponding alcohols with the diastereomer (anti/syn) ratio of 53/47. The enantiomer excesses of anti-(2S, 3S)- and syn-(2S, 3R)-hydroxy esters were 89 and > 99ee% respectively. C. vulgaris and C. regularis afforded
The total synthesis of chlorotonil A.
Rahn N and Kalesse M
Angewandte Chemie (International Edition in English), 47(3), 597-599 (2008)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico