E26258
Ethylene carbonate
98%
Sinónimos:
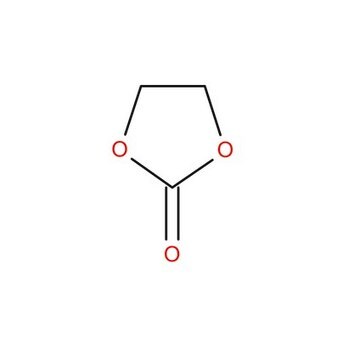
1,3-Dioxolan-2-one
About This Item
Productos recomendados
vapor density
3.04 (vs air)
Quality Level
vapor pressure
0.02 mmHg ( 36.4 °C)
assay
98%
bp
243-244 °C/740 mmHg (lit.)
mp
35-38 °C (lit.)
density
1.321 g/mL at 25 °C (lit.)
SMILES string
O=C1OCCO1
InChI
1S/C3H4O3/c4-3-5-1-2-6-3/h1-2H2
InChI key
KMTRUDSVKNLOMY-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- In the synthesis of aliphatic polyurethanes using diamines and diols.
- As a precursor for ring-opening polymerization using KOH as initiator.
- As a reactant in the preparation of dimethyl carbonate, glycerol carbonate by transesterification reaction.
Related product
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 Oral
target_organs
Kidney
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
289.4 °F - closed cup
flash_point_c
143 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Solid-state lithium fast-ion conductors are crucial for safer, high-energy-density all-solid-state batteries, addressing conventional battery limitations.
Solid-state lithium fast-ion conductors are crucial for safer, high-energy-density all-solid-state batteries, addressing conventional battery limitations.
Solid-state lithium fast-ion conductors are crucial for safer, high-energy-density all-solid-state batteries, addressing conventional battery limitations.
Solid-state lithium fast-ion conductors are crucial for safer, high-energy-density all-solid-state batteries, addressing conventional battery limitations.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico