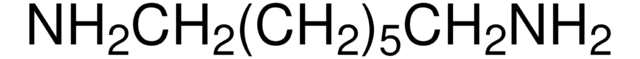D23807
1,3-Diaminopropane dihydrochloride
98%
Sinónimos:
1,3-Propanediamine, 1,3-Propanediamine dihydrochloride
About This Item
Productos recomendados
assay
98%
form
powder
mp
246-250 °C (lit.)
SMILES string
Cl[H].Cl[H].NCCCN
InChI
1S/C3H10N2.2ClH/c4-2-1-3-5;;/h1-5H2;2*1H
InChI key
HYOCSVGEQMCOGE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Tetradentate bis-phosphine ligands (P(2)N(2) and P(2)S(2)) and their Rh(III), Ni(II) and (105)Rh complexes: X-ray crystal structures of trans-[RhCl(2)(L2)]PF(6), [Ni(L2)](PF(6))(2) and μ-O(2)SO(2)-[Ni(L5)](2)(PF(6))(2).: This study explores the synthesis and characterization of tetradentate bis-phosphine ligands and their complexes, demonstrating significant advancements in ligand design and potential applications in catalysis and medicinal chemistry (Cagnolini et al., 2011).
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico