ALD00500
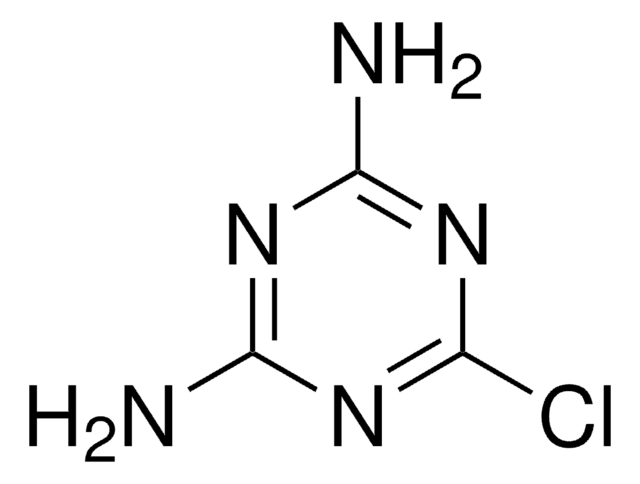
5-Methoxy-1,2,3-triazine
About This Item
Productos recomendados
form
powder
Quality Level
storage temp.
2-8°C
SMILES string
COC1=CN=NN=C1
InChI
1S/C4H5N3O/c1-8-4-2-5-7-6-3-4/h2-3H,1H3
InChI key
HVBZCUMRMKODNE-UHFFFAOYSA-N
General description
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Inverse electron demand Diels-Alder reactions enable total synthesis of natural products with heteroaromatic ring systems.
Contenido relacionado
As the exploration of the properties of complex natural products becomes increasingly more sophisticated with the technological advances being made in their screening and evaluation and as structural details of their interaction with biological targets becomes more accessible, the importance and opportunities for providing unique solutions to complex biological problems has grown. The Boger Lab addresses these challenging problems by understanding the complex solutions and subtle design elements that nature has provided in the form of a natural product and work to extend the solution through rational design elements to provide more selective, more efficacious, or more potent agents designed specifically for the problem or target under investigation. The resulting efforts have reduced many difficult or intractable synthetic challenges to manageable problems providing an approach not only to the natural product but one capable of simple extrapolation to a series of structural analogs with improved selectivity and efficacy.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico