A50606
6-Aminouracil
97%
Sinónimos:
4-Amino-2,6-dihydroxypyrimidine, 6-Amino-2,4-pyrimidinediol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C4H5N3O2
Número de CAS:
Peso molecular:
127.10
Beilstein/REAXYS Number:
120491
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
powder
mp
≥360 °C (lit.)
SMILES string
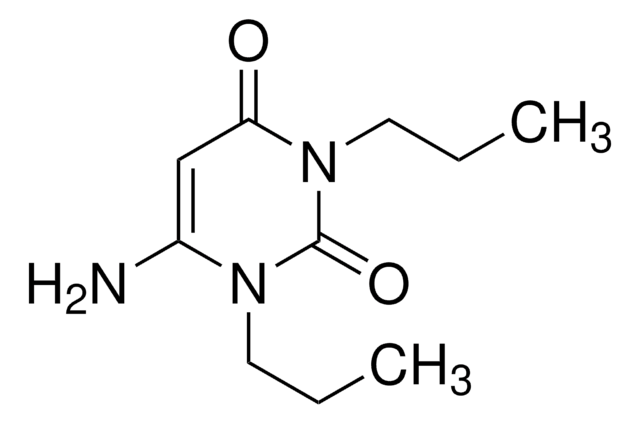
Nc1cc(O)nc(O)n1
InChI
1S/C4H5N3O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H4,5,6,7,8,9)
InChI key
LNDZXOWGUAIUBG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
María-Jesús Pérez-Pérez et al.
Mini reviews in medicinal chemistry, 5(12), 1113-1123 (2005-12-27)
Thymidine Phosphorylase (TPase) catalyses the reversible phosphorolysis of pyrimidine 2'-deoxynucleosides to 2-deoxyribose-1-phosphate and their respective pyrimidine bases, including the phosphorolysis of nucleoside analogues with important antiviral or anticancer properties. Moreover, TPase, identified also as the angiogenic platelet-derived endothelial cell growth
H Sladowska et al.
Acta poloniae pharmaceutica, 53(1), 39-46 (1996-01-01)
Synthesis of 6-substituted 2,4-dioxo-1,2,3,4,5,6,7,8-octahydropyrimido[4,5-d]pyrimidines [III-VI] obtained by cyclocondensation of 1-phenyl-6-aminouracil with formaline and the primary amines is described. Compounds III, V, VI in the Mannich reaction with secondary cyclic amines yield the corresponding 3-substituted N-aminomethyl derivatives VII-X. Some of them
P M Tarantino et al.
Journal of medicinal chemistry, 42(11), 2035-2040 (1999-06-04)
6-Anilinouracils (6-AUs) are dGTP analogues which selectively inhibit the DNA polymerase III of Bacillus subtilis and other Gram-positive bacteria. To enhance the potential of the 6-AUs as antimicrobial agents, a structure-activity relationship was developed involving substitutions of the uracil N3
A Maldonado et al.
Experimental cell research, 161(1), 172-180 (1985-11-01)
We have approached the study of the ability of different types of lesions produced by DNA-damaging agents to develop sister-chromatid exchanges (SCEs) by analyzing SCE levels observed in Allium cepa L cells with BrdU-substituted DNA and exposed to visible light
Akos Bányász et al.
The journal of physical chemistry. B, 114(39), 12708-12719 (2010-09-14)
A detailed experimental and computational study of the absorption and fluorescence spectra of 5-aminouracil (5 AU) and 6-aminouracil (6 AU) in aqueous solution is reported. The lowest energy band of the steady-state absorption spectra of 5 AU is considerably red-shifted
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico