923206
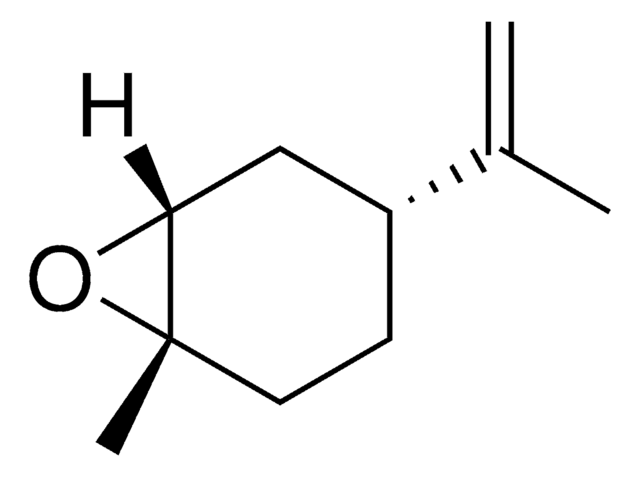
trans-(-)-limonene oxide
≥95%
Sinónimos:
(1R,4S,6S)-4-isopropenyl-1-methyl-7-oxabicyclo[4.1.0]heptane
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H16O
Número de CAS:
Peso molecular:
152.23
MDL number:
UNSPSC Code:
12352212
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥95%
form
liquid
refractive index
n/D 1.467
density
0.920 g/mL
functional group
ether
InChI
1S/C10H16O/c1-7(2)8-4-5-10(3)9(6-8)11-10/h8-9H,1,4-6H2,2-3H3/t8-,9-,10+/m0/s1
InChI key
CCEFMUBVSUDRLG-LPEHRKFASA-N
Application
trans-(-)-Limonene oxide is a valuable chiral terpene oxide building block.Used in the synthesis of Phosphorus Incorporation (PI) reagents, as a biologically sourced component of polycarbonate polymers, and as a building block for synthetic applications requiring a reactive epoxide or alkene.
related product
Referencia del producto
Descripción
Precios
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Samantha A Green et al.
Journal of the American Chemical Society, 141(19), 7709-7714 (2019-04-30)
Metal-hydride hydrogen atom transfer (MHAT) functionalizes alkenes with predictable branched (Markovnikov) selectivity. The breadth of these transformations has been confined to π-radical traps; no sp3 electrophiles have been reported. Here we describe a Mn/Ni dual catalytic system that hydroalkylates unactivated
Synthesis and self-assembly of biobased poly(limonene carbonate)-block-poly(cyclohexene carbonate) diblock copolymers prepared by sequential ring-opening copolymerization.
Bailer J, et al.
Green Chemistry, 21, 2266-2272 (2019)
Ke-Yin Ye et al.
Journal of the American Chemical Society, 141(24), 9548-9554 (2019-06-11)
Organic radicals are generally short-lived intermediates with exceptionally high reactivity. Strategically, achieving synthetically useful transformations mediated by organic radicals requires both efficient initiation and selective termination events. Here, we report a new catalytic strategy, namely, bimetallic radical redox-relay, in the
Julian C Lo et al.
Journal of the American Chemical Society, 139(6), 2484-2503 (2017-01-18)
This Article details the development of the iron-catalyzed conversion of olefins to radicals and their subsequent use in the construction of C-C bonds. Optimization of a reductive diene cyclization led to the development of an intermolecular cross-coupling of electronically-differentiated donor
Dongmin Xu et al.
Journal of the American Chemical Society, 142(12), 5785-5792 (2020-02-29)
Phosphorus Incorporation (PI, abbreviated Π) reagents for the modular, scalable, and stereospecific synthesis of chiral phosphines and methylphosphonate nucleotides are reported. Synthesized from trans-limonene oxide, this reagent class displays an unexpected reactivity profile and enables access to chemical space distinct
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico