915793
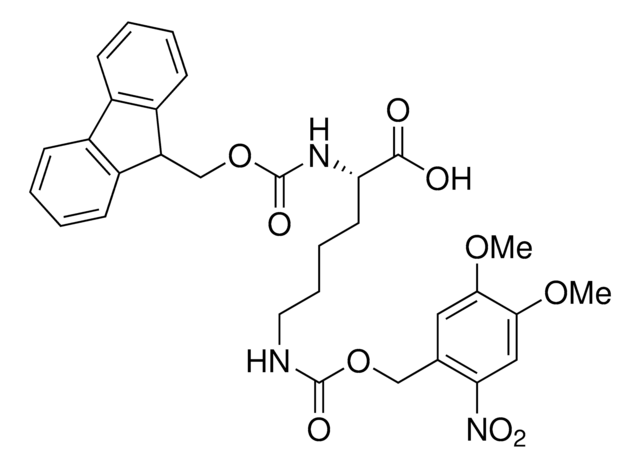
Methyl-o-nitropiperonyllysine
≥95%
Sinónimos:
N6-((1-(6-Nitrobenzo[d][1,3]dioxol-5-yl)ethoxy)carbonyl)-L-lysine, Light-triggered decaging Lys, Photo-controlled amino acid, Photocaged amino acid, Photocleavable lysine derivative, mNPK
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C16H21N3O8
Número de CAS:
Peso molecular:
383.35
MDL number:
UNSPSC Code:
12352209
NACRES:
NA.22
Productos recomendados
Application
Methyl-o-nitropiperonyllysine (mNPK) trifluoroacetic acid is a photo-responsive unnatural amino acid (UAA) for spatiotemporal control of biological molecules or processes as reported by Kneuttinger et al. Irradiation with UV light decages the Lys amino acid, freeing the residue or protein for biological activity. Tools such as mNPK will find wide utility in light regulation of activity, allostery, and enzyme pathways.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Light Regulation of Enzyme Allostery through Photoresponsive Unnatural Amino Acids
Precise Photoremovable Perturbation of a Virus-Host Interaction
Genetic code expansion in the mouse brain
Genetically encoded optical activation of DNA recombination in human cells
Bioorthogonal Chemical Activation of Kinases in Living Systems
Precise Photoremovable Perturbation of a Virus-Host Interaction
Genetic code expansion in the mouse brain
Genetically encoded optical activation of DNA recombination in human cells
Bioorthogonal Chemical Activation of Kinases in Living Systems
Related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
James Hemphill et al.
Journal of the American Chemical Society, 135(36), 13433-13439 (2013-08-13)
Photocaging provides a method to spatially and temporally control biological function and gene expression with high resolution. Proteins can be photochemically controlled through the site-specific installation of caging groups on amino acid side chains that are essential for protein function.
Arnaud Gautier et al.
Journal of the American Chemical Society, 132(12), 4086-4088 (2010-03-12)
Precise photochemical control of protein function can be achieved through the site-specific introduction of caging groups. Chemical and enzymatic methods, including in vitro translation and chemical ligation, have been used to photocage proteins in vitro. These methods have been extended
Olivia S Walker et al.
Journal of the American Chemical Society, 138(3), 718-721 (2016-01-14)
Isocitrate dehydrogenase is mutated at a key active site arginine residue (Arg172 in IDH2) in many cancers, leading to the synthesis of the oncometabolite (R)-2-hydroxyglutarate (2HG). To investigate the early events following acquisition of this mutation in mammalian cells we
Arnaud Gautier et al.
Journal of the American Chemical Society, 133(7), 2124-2127 (2011-01-29)
We report a general strategy for creating protein kinases in mammalian cells that are poised for very rapid activation by light. By photoactivating a caged version of MEK1, we demonstrate the specific, rapid, and receptor independent activation of an artificial
Sarah B Erickson et al.
Angewandte Chemie (International ed. in English), 56(15), 4234-4237 (2017-03-16)
Viruses utilize distinct binding interactions with a variety of host factors to gain entry into host cells. A chemical strategy is described to precisely perturb a specific molecular interaction between adeno-associated virus and its host cell, which can be rapidly
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico