900624
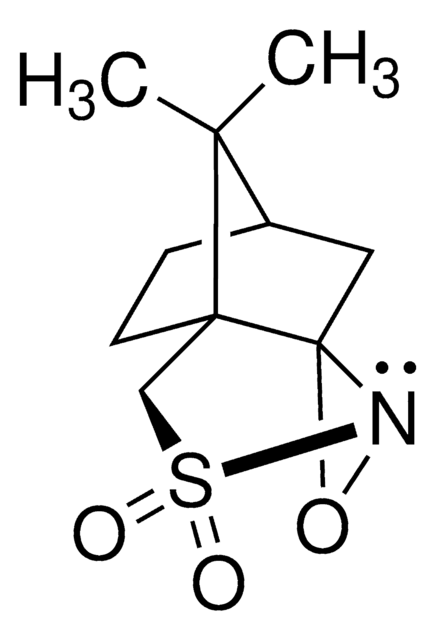
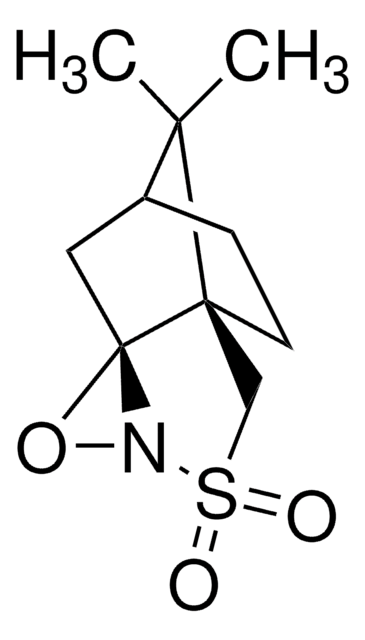
Di-t-butyl oxaziridine
≥95%
Sinónimos:
Kurti oxaziridine
About This Item
Productos recomendados
Quality Level
assay
≥95%
form
liquid
availability
available only in USA
refractive index
n/D 1.4453
density
0.90 g/mL
storage temp.
2-8°C
SMILES string
CC(C)(C)C1(NO1)C(C)(C)C
Application
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
143.6 °F
flash_point_c
62 °C
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Contenido relacionado
Amines and their derivatives are ubiquitous substances since they make up the overwhelming majority of drug molecules, agrochemicals as well as many compounds that are produced by plants and living organisms (i.e., natural products).
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico