759902
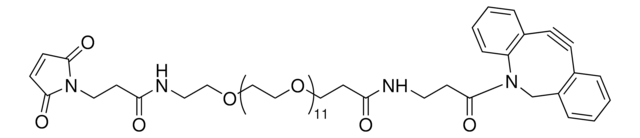
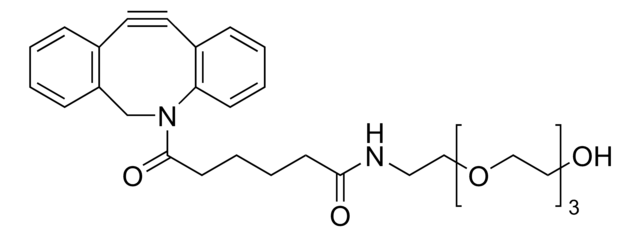
Dibenzocyclooctyne-PEG4-acid
for Copper-free Click Chemistry
Sinónimos:
Polyethylene glycol, DBCO-PEG4-Acid
About This Item
Productos recomendados
Quality Level
form
solid
reaction suitability
reaction type: click chemistry
reagent type: linker
functional group
carboxylic acid
storage temp.
−20°C
SMILES string
O=C(CCCCC(NCCOCCOCCOCCOCCC(O)=O)=O)N1CC2=C(C=CC=C2)C#CC3=C1C=CC=C3
InChI
1S/C32H40N2O8/c35-30(33-16-18-40-20-22-42-24-23-41-21-19-39-17-15-32(37)38)11-5-6-12-31(36)34-25-28-9-2-1-7-26(28)13-14-27-8-3-4-10-29(27)34/h1-4,7-10H,5-6,11-12,15-25H2,(H,33,35)(H,37,38)
InChI key
RMYANOWYMFCGGS-UHFFFAOYSA-N
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)

![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)