718149
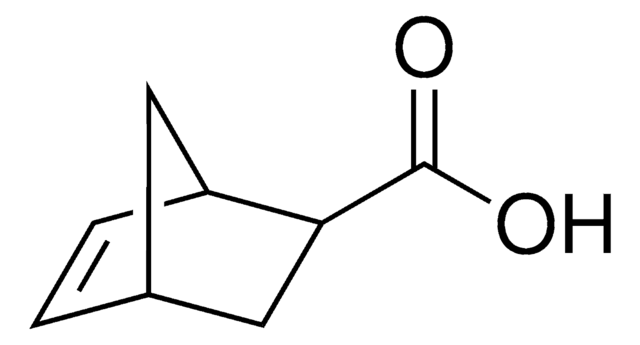
exo-5-Norbornenecarboxylic acid
97%
Sinónimos:
(1R,2S,4R)-Bicyclo[2.2.1]hept-5-ene-2-carboxylic acid, NC
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H10O2
Número de CAS:
Peso molecular:
138.16
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Productos recomendados
Quality Level
assay
97%
form
solid
mp
40-44 °C
SMILES string
OC(=O)[C@@H]1C[C@@H]2C[C@H]1C=C2
InChI
1S/C8H10O2/c9-8(10)7-4-5-1-2-6(7)3-5/h1-2,5-7H,3-4H2,(H,9,10)/t5-,6+,7+/m0/s1
InChI key
FYGUSUBEMUKACF-RRKCRQDMSA-N
General description
Exo-5-norbornenecarboxylic acid is a bicyclic compound that has potential applications in the field of material science due to its unique chemical properties. It is a versatile building block for the synthesis of various functional materials, including polymers, dendrimers, and self-assembled monolayers. It can be used to modify surfaces or to functionalize nanoparticles, influencing their optical, magnetic, or electronic properties. It is also often used in ring-opening metathesis polymerization reactions to form polymers with controlled molecular weight and structure. Additionally, exo-5-norbornenecarboxylic acid can function as a ligand for coordination chemistry and catalysis.
Application
Exo-5-norbornenecarboxylic acid can be used as:
- A starting material in the synthesis of the metathesis polymer via a ring-opening metathesis polymerization (ROMP) reaction of the ester of exo-5-norbornenecarboxylic acid and 1,1′-bi-2-naphthol.
- A monomer in the preparation of thin films via surface-initiated polymerization process. The resulting thin film serves as a template for selective deposition and etching of metal oxides, which is of significant importance in the microelectronic industry.
- Different crosslinkers for ring-opening metathesis polymerization.
- Norbornene-functionalized monomers, which are used to make poly(norbornene)s via ring-opening metathesis polymerization (ROMP).
- Hydrolytically cleavable and hydrolytically stable functionalized macromonomers for hydrogel preparation.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
>230.0 °F
flash_point_c
> 110 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Jürgen Herrler et al.
Magnetic resonance in medicine, 85(6), 3140-3153 (2021-01-06)
To mitigate spatial flip angle (FA) variations under strict specific absorption rate (SAR) constraints for ultra-high field MRI using a combination of universal parallel transmit (pTx) pulses and fast subject-specific optimization. Data sets consisting of B0 , B 1 +
Wenjun Zheng et al.
BMC structural biology, 9, 45-45 (2009-07-14)
It is increasingly recognized that protein functions often require intricate conformational dynamics, which involves a network of key amino acid residues that couple spatially separated functional sites. Tremendous efforts have been made to identify these key residues by experimental and
Christopher L McGann et al.
Macromolecular bioscience, 16(1), 129-138 (2015-10-06)
A range of chemical strategies have been used for crosslinking recombinant polypeptide hydrogels, although only a few have employed photocrosslinking approaches. Here, we capitalize on the novel insect protein, resilin, and the versatility of click reactions to introduce a resilin-like
Nilwala Kottegoda et al.
ACS nano, 11(2), 1214-1221 (2017-01-26)
While slow release of chemicals has been widely applied for drug delivery, little work has been done on using this general nanotechnology-based principle for delivering nutrients to crops. In developing countries, the cost of fertilizers can be significant and is
Patricia Gumbley et al.
Macromolecular rapid communications, 34(23-24), 1838-1843 (2013-11-12)
This communication describes photoresponsive gels, prepared using ring-opening metathesis polymerization (ROMP), that dissolve upon irradiation with ultraviolet light. Exposure of mixtures of norbornene-type ROMP monomers and new photoreactive cross-linkers comprising two norbornene units bound through a chain containing o-nitrobenzyl esters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico