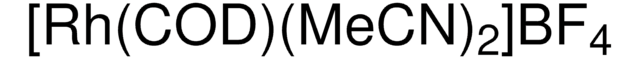695467
1,3,5-Triaza-7-phosphatricyclo[3.3.1.13,7]decane
97%
Sinónimos:
1,3,5-Triaza-7-phosphaadamantane, NSC 266642, PTA
About This Item
Productos recomendados
Quality Level
assay
97%
form
solid
reaction suitability
reagent type: ligand
reaction type: Hydroformylations
reagent type: ligand
reaction type: Hydrogenations
reagent type: ligand
reaction type: Morita-Baylis-Hillman Reactions
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
244-250 °C
functional group
phosphine
SMILES string
C1N2CN3CN1CP(C2)C3
InChI
1S/C6H12N3P/c1-7-2-9-3-8(1)5-10(4-7)6-9/h1-6H2
InChI key
FXXRPTKTLVHPAR-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- The molecular mechanisms of antimetastatic ruthenium compounds explored through DIGE proteomics.: This study examines the antimetastatic properties of ruthenium compounds using DIGE proteomics. The involvement of 1,3,5-Triaza-7-phosphaadamantane in the complexation with ruthenium and its biological effects were analyzed, highlighting its potential in anticancer therapies (Guidi et al., 2013).
- Synthesis, antimicrobial and antiproliferative activity of novel silver(I) tris(pyrazolyl)methanesulfonate and 1,3,5-triaza-7-phosphadamantane complexes.: This research details the synthesis of novel silver complexes containing 1,3,5-Triaza-7-phosphaadamantane, evaluating their antimicrobial and antiproliferative activities, which demonstrates the compound′s utility in biomedical applications (Pettinari et al., 2011).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Amines and phosphines in nucleophilic catalysis are discussed, addressing the air sensitivity of phosphines.
Contenido relacionado
The Frost group has a longstanding interest in aqueous phase organometallic chemistry with an emphasis on water-soluble phosphine ligands. One focus of this research group has been centered on the chemistry of the neutral, air stable, and water-soluble heterocyclic phosphine 1,3,5-Triaza-7-phosphaadamantane (PTA). PTA is a small phosphine ligands with a cone angle of ~103°. PTA binds metal centers quite strongly and is electronically more donating than PPh3. Ruthenium complexes of PTA have exhibited impressive anticancer activity. A wide variety of transition metal complexes of PTA have been utilized as catalysts for reactions such as hydrogenation, C-C bond forming reactions, atom transfer radical addition, and nitrile hydration.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
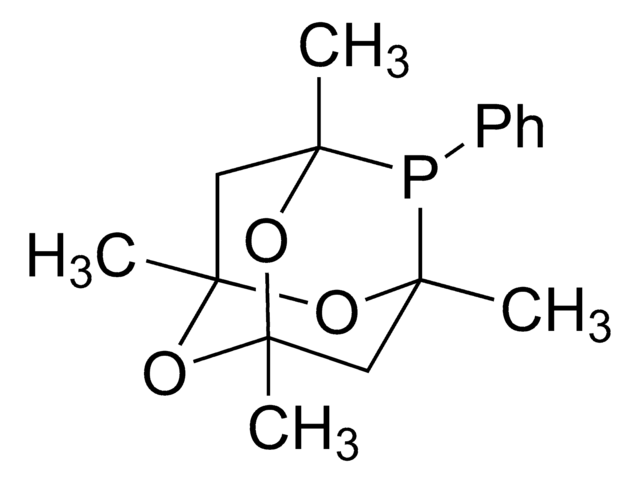
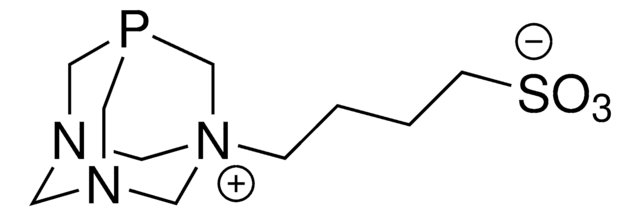
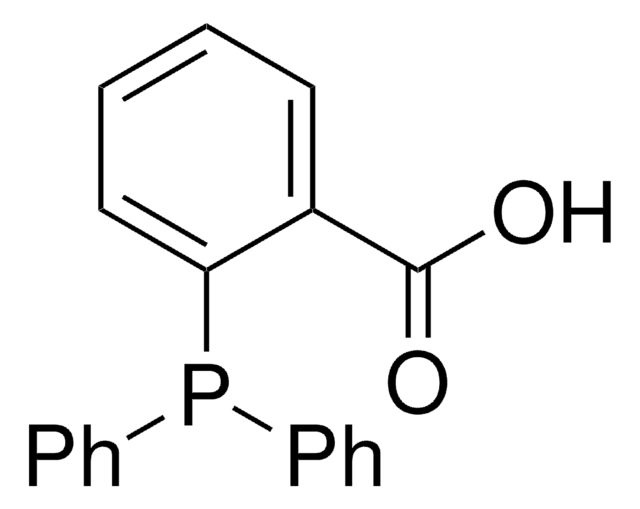

![3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane 97%](/deepweb/assets/sigmaaldrich/product/structures/198/979/42d0b946-b026-4831-b284-fcb0e91533d9/640/42d0b946-b026-4831-b284-fcb0e91533d9.png)
![1,4-Bis[(phenyl-3-propanesulfonate) phosphine] butane disodium salt](/deepweb/assets/sigmaaldrich/product/structures/322/102/cc3c448f-049a-41a4-93c3-26d70302d06d/640/cc3c448f-049a-41a4-93c3-26d70302d06d.png)


![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)
![2,8,9-Triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane 97%](/deepweb/assets/sigmaaldrich/product/structures/750/287/cc77a98e-fa6c-4d81-9f3e-f392770724ac/640/cc77a98e-fa6c-4d81-9f3e-f392770724ac.png)