63680
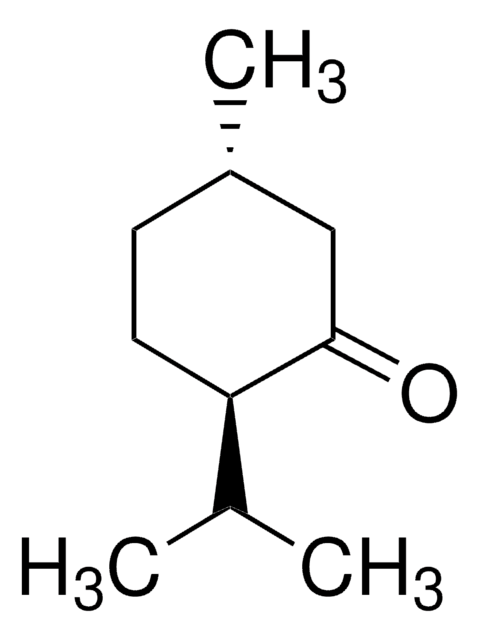
Menthone
mixture of isomers, ≥97.0% (GC)
Sinónimos:
2-Isopropyl-5-methylcyclohexanone
About This Item
Productos recomendados
assay
≥97.0% (GC)
bp
85-88 °C/12 mmHg (lit.)
density
0.896 g/mL at 20 °C (lit.)
functional group
ketone
SMILES string
CC(C)C1CCC(C)CC1=O
InChI
1S/C10H18O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-9H,4-6H2,1-3H3
InChI key
NFLGAXVYCFJBMK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- To prepare the model flavor mix in a study to investigate the release of various aroma compounds in xanthan-thickened food model systems having different viscosities.
- As standard in the quantification of volatile constituents and odour-activity value (OAV) in ′Marion′ and ′Black Diamond′.
- Modified semisolid agar antifungal susceptibility method (SAAS) to investigate the anti-fungal activities of various cyclic terpenes against Fusarium verticillioides MRC 826.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Resp. Sens. 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
156.2 °F - closed cup
flash_point_c
69 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond adjacent to a carbonyl, which converts the ketones to esters and the cyclic ketones to lactones.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico