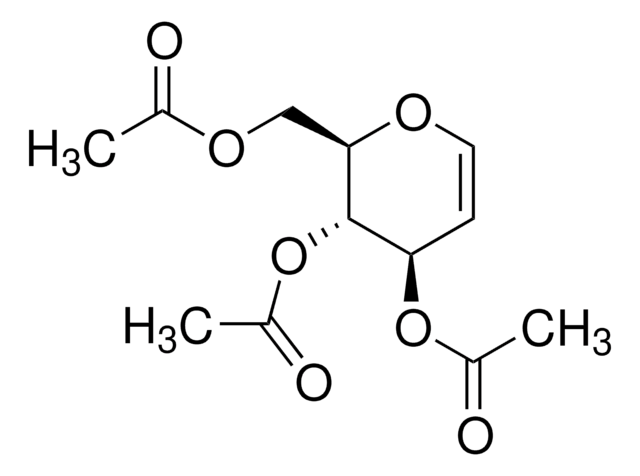
464058
D-Glucal
96%
Sinónimos:
1,5-Anhydro-2-deoxy-D-arabino-hex-1-enitol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H10O4
Número de CAS:
Peso molecular:
146.14
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
96%
form
solid
optical activity
[α]21/D −7°, c = 1.9 in H2O
impurities
<1% methyl alcohol
mp
58-60 °C (lit.)
storage temp.
2-8°C
SMILES string
OC[C@H]1OC=C[C@@H](O)[C@@H]1O
InChI
1S/C6H10O4/c7-3-5-6(9)4(8)1-2-10-5/h1-2,4-9H,3H2/t4-,5-,6+/m1/s1
InChI key
YVECGMZCTULTIS-PBXRRBTRSA-N
Categorías relacionadas
Application
Important building block for both solution- and solid-phase synthesis of oligosaccharides.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
The role of pyridoxal 5'-phosphate and orthophosphate in general acid-base catalysis by alpha-glucan phosphorylases.
H W Klein et al.
Progress in clinical and biological research, 144A, 147-160 (1984-01-01)
Qi Chen et al.
Carbohydrate research, 342(11), 1405-1411 (2007-05-23)
A total synthesis of the 12-membered ring natural macrolide, sporiolide B, was achieved from D-glucal in 17 steps with 4.8% overall yield. The required stereochemical configuration at C-3 and C-5 in sporiolide B was easily introduced by applying a Mitsunobu
H W Klein et al.
Biochemistry, 21(26), 6675-6684 (1982-12-21)
D-Glucal, containing a highly reactive double bond, can replace glucose 1-phosphate as the glucosyl donor in phosphorylase-catalyzed glucosyl transfer to a suitable oligo- or polysaccharide acceptor: D-glucal + Pi + (glucose)Pi leads to n 2-deoxy-alpha-D-glucosyl(glucose)n in equilibrium 2-deoxy-alpha-D-glucose-1-P + (glucose)n.
Patricia Wildberger et al.
Carbohydrate research, 356, 224-232 (2012-05-18)
Cellobiose phosphorylase from Cellulomonas uda (CuCPase) is shown to utilize D-glucal as slow alternative donor substrate for stereospecific glycosyl transfer to inorganic phosphate, giving 2-deoxy-α-D-glucose 1-phosphate as the product. When performed in D(2)O, enzymatic phosphorolysis of D-glucal proceeds with incorporation
S Chiba et al.
Biochemistry, 27(5), 1464-1469 (1988-03-08)
Alpha-Glucosidases from Aspergillus niger, pig serum, ungerminated rice, buckwheat, and sugar beet seeds (but not from brewers' yeast or honeybee) were found to catalyze the hydration of D-glucal. Each reactive alpha-glucosidase, incubated with D-glucal in D2O, was shown to protonate
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico