444286
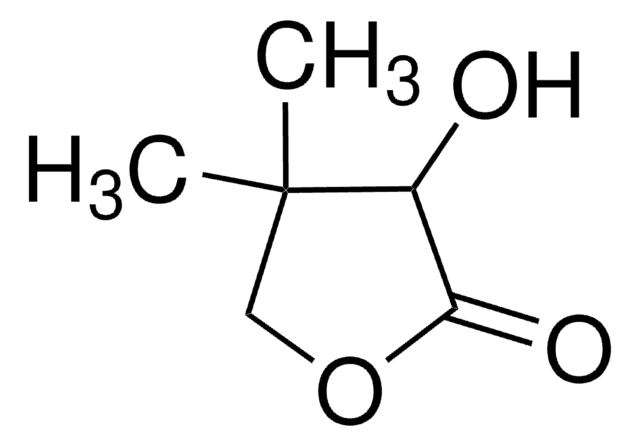
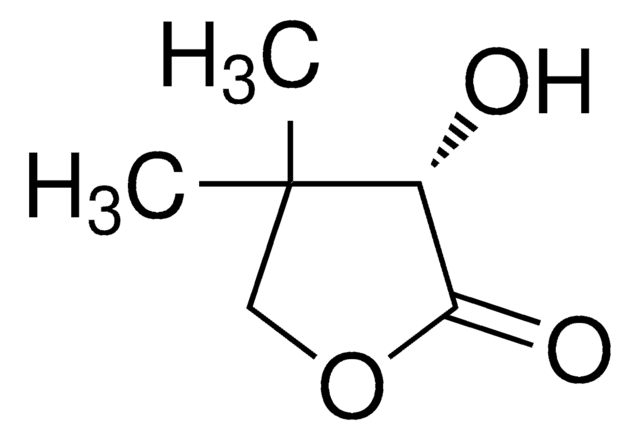
(R)-(+)-α-Hydroxy-γ-butyrolactone
95%, optical purity ee: 98% (GLC)
Sinónimos:
(R)-4,5-Dihydro-3-hydroxy-2(3H)-furanone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C4H6O3
Número de CAS:
Peso molecular:
102.09
Beilstein/REAXYS Number:
80588
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
95%
form
liquid
optical activity
[α]23/D +66°, c = 1.15 in chloroform
optical purity
ee: 98% (GLC)
refractive index
n20/D 1.467 (lit.)
bp
133 °C/10 mmHg (lit.)
density
1.309 g/mL at 25 °C (lit.)
SMILES string
O[C@@H]1CCOC1=O
InChI
1S/C4H6O3/c5-3-1-2-7-4(3)6/h3,5H,1-2H2/t3-/m1/s1
InChI key
FWIBCWKHNZBDLS-GSVOUGTGSA-N
Application
(R)-(+)-α-Hydroxy-γ-butyrolactone can be used as a starting material to synthesize:
- δ-Azaproline by reacting with benzyloxycarbonyl aminophthalimide via Mitsunobu reactions.
- Homochiral (R)-2,4-dihydroxybutyramide seco-pseudonucleoside reagents.
- Botryolide B via esterification and ring-closing metathesis reaction.
- Pregnane derivatives containing γ-butyrolactones as potential glucocorticoid agonists.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Novel glucocorticoid antedrugs possessing a 21-(?-lactone) ring.
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 6, 831-839 (2002)
Concise total synthesis of botryolide B
Mohapatra DK, et al.
Royal Society of Chemistry Advances, 4(16), 8335-8340 (2014)
Novel glucocorticoid antedrugs possessing a 21-(γ-lactone) ring
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 4(6), 831-839 (2002)
Xiaohui Gou et al.
Frontiers in physiology, 11, 686-686 (2020-07-17)
Dentin sialoprotein (DSP), the NH2-terminal fragment of dentin sialophosphoprotein (DSPP), is essential for dentin formation and further processed into small fragments inside the odontoblasts. Gelatinases, including matrix metalloproteinases 9 (MMP9) and MMP2, were able to cleave DSP(P) in tooth structures.
Natalia N Dioubankova et al.
Organic letters, 4(26), 4607-4610 (2002-12-20)
[reaction: see text] Two series of seco-pseudonucleoside synthons were synthesized from (R)-(+)-alpha-hydroxy-gamma-butyrolactone and (R)-(-)-pantolactone by aminolysis, side-chain protection, dimethoxytritylation, and phosphitylation or solid-phase attachment. The phosphoramidites and solid supports were used in automated DNA synthesis to prepare oligonucleotides modified with
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico