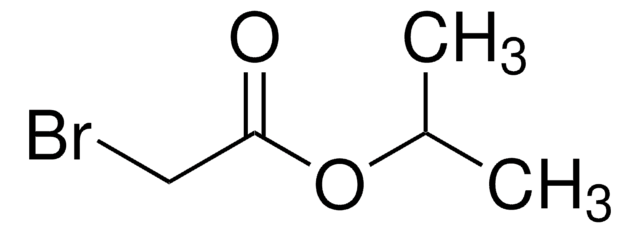
404276
Phenyl bromoacetate
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
BrCH2CO2C6H5
Número de CAS:
Peso molecular:
215.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
solid
bp
134 °C/15 mmHg (lit.)
mp
31-33 °C (lit.)
density
1.508 g/mL at 25 °C (lit.)
functional group
bromo
ester
phenoxy
SMILES string
BrCC(=O)Oc1ccccc1
InChI
1S/C8H7BrO2/c9-6-8(10)11-7-4-2-1-3-5-7/h1-5H,6H2
InChI key
UEWYUCGVQMZMGY-UHFFFAOYSA-N
General description
Phenyl bromoacetate is an aromatic ester.
Application
Phenyl bromoacetate may be employed as alkylation reagent in the preparation of 2-(phenoxycarbonyl)methyl triazoles. It may be used in the synthesis of the A-ring of cylindrospermopsin. It may be used in the synthesis of the following 4-thiazolidinones:
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-1,3-thiazolidin-4-one
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-3-methyl-1,3-thiazolidin-4-one
- 3-ethyl-2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-1,3-thiazolidin-4-one
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene] hydrazinyl}-3-phenyl-1,3-thiazolidin-4-one
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Robert M Williams et al.
ACS symposium series. American Chemical Society, 1009, 420-442 (2010-06-22)
We report the application of diphenyloxazinone glycinate chiral templates to asymmetric syntheses of cylindrospermospin, 7-epi-cylindrospermopsin, 7-deoxycylindrospermopsin, and spirotryprostatins A and B. Synthetic studies toward quinine, nakadomarin A, and palau'amine using these templates are also described.
Duen-Ren Hou et al.
Bioorganic & medicinal chemistry letters, 19(3), 1022-1025 (2008-12-20)
This letter reports the new entry of novel 1,2,3-triazole derivatives as CB1 receptor antagonists. The design, synthesis and biological evaluation of N1 and N2 substituted 1,2,3-trizoles are described. The N2 substituted, symmetrical 1,2,3-triazoles are more potent ligands than the unsymmetrical
Synthesis and Antimicrobial Activity Evaluation of Novel 4-Thiazolidinones Containing a Pyrone Moiety.
Nechak R, et al.
Synthetic Communications, 1-11 (2014)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico