400173
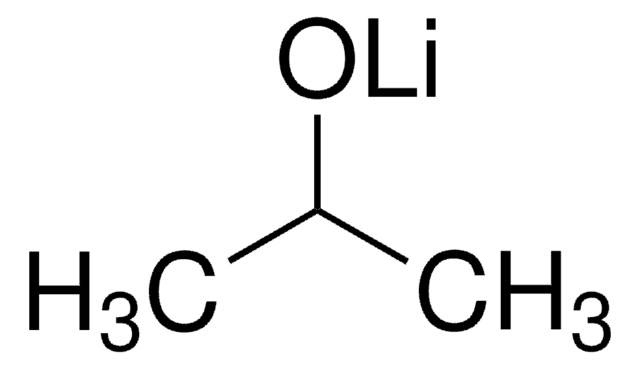
Lithium tert-butoxide
97%
Sinónimos:
LiOtBu
About This Item
Productos recomendados
Quality Level
assay
97%
form
powder and chunks
SMILES string
[Li+].CC(C)(C)[O-]
InChI
1S/C4H9O.Li/c1-4(2,3)5;/h1-3H3;/q-1;+1
InChI key
LZWQNOHZMQIFBX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
Other synthetic applications:
- In combination with potassium diisopropylamide, LiOtBu can be used to deprotonate 1-(phenylseleno) alkenes and bis (phenylseleno) acetals.
- LiOtBu can mediate the α-alkylation reaction of ketones with primary alcohols in the absence of any transition metal catalyst.
- LiOtBu is an effective base for the synthesis of 3,4,5-trisubstituted 3H-oxazol-2-ones and 3,4-disubstituted (Z)-oxazolidin-2-ones from substituted propargyl alcohols and aryl/alkyl isocyanates using DMF as a solvent.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Self-heat. 1 - Skin Corr. 1B
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Few Monolayer Atomic Layer Deposition (ALD) on Surfaces and Interfaces for Energy Applications
Nanomaterials are considered a route to the innovations required for large-scale implementation of renewable energy technologies in society to make our life sustainable.
Nanomaterials are considered a route to the innovations required for large-scale implementation of renewable energy technologies in society to make our life sustainable.
Nanomaterials are considered a route to the innovations required for large-scale implementation of renewable energy technologies in society to make our life sustainable.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico