399361
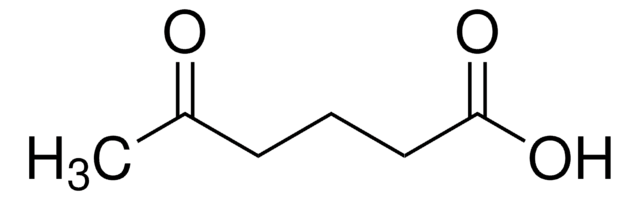
6-Oxoheptanoic acid
technical grade, 90%
About This Item
Productos recomendados
grade
technical grade
Quality Level
assay
90%
form
solid
bp
158-162 °C/9 mmHg (lit.)
mp
35-37 °C (lit.)
density
1.059 g/mL at 25 °C (lit.)
functional group
carboxylic acid
ketone
SMILES string
CC(=O)CCCCC(O)=O
InChI
1S/C7H12O3/c1-6(8)4-2-3-5-7(9)10/h2-5H2,1H3,(H,9,10)
Inchi Key
IZOQMUVIDMLRDC-UHFFFAOYSA-N
Categorías relacionadas
General description
Application
- As ketone linker used for the conjugation of hydrazide derivatives to proteins.
- Synthesis of N-(2-propynyl)-6-oxoheptanamide.
- Synthesis of adenosine triphosphate derivative.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico