393622
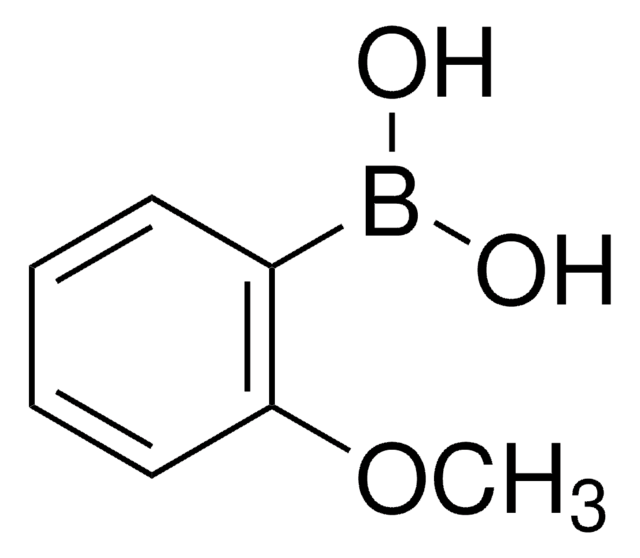
p-Tolylboronic acid
97%
Sinónimos:
(p-Methylphenyl)boronic acid, 4-Methylbenzeneboronic acid, 4-Methylphenylboronic acid, 4-Tolueneboronic acid, 4-Tolylboronic acid, p-Tolueneboronic acid, NSC 62870, p-Methylbenzeneboronic acid
About This Item
Productos recomendados
Nivel de calidad
Ensayo
97%
mp
256-263 °C (lit.)
cadena SMILES
Cc1ccc(cc1)B(O)O
InChI
1S/C7H9BO2/c1-6-2-4-7(5-3-6)8(9)10/h2-5,9-10H,1H3
Clave InChI
BIWQNIMLAISTBV-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- Palladium (Pd)-catalyzed direct arylation
- Direct Palladium(II)-Catalyzed Synthesis
- Palladium-catalyzed arylation by Suzuki-Miyaura cross-coupling in water
- Cyclopalladation
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Ruthenium catalyzed direct arylation
- Rhodium-catalyzed asymmetric conjugate addition
- Ligand-free copper-catalyzed cross-coupling reactions
- Regioselective arylation and alkynylation by Suzuki-Miyaura and Sonogashira cross-coupling reactions
- Ligand-free Suzuki, Sonogashira, and Heck cross-coupling reactions
Reagent used in Preparation of
- Catalysts for Suzuki-Miyaura cross-coupling of aryl bromides
- Recyclable Palladium nanoparticle catalysts immobilized by click ionic copolymers as for Suzuki-Miyaura cross-coupling reactions in water
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
MIDA-protected boronate esters offer stability, chromatography compatibility, and reactivity in anhydrous cross-coupling conditions.
MIDA-protected boronate esters offer stability, chromatography compatibility, and reactivity in anhydrous cross-coupling conditions.
MIDA-protected boronate esters offer stability, chromatography compatibility, and reactivity in anhydrous cross-coupling conditions.
MIDA-protected boronate esters offer stability, chromatography compatibility, and reactivity in anhydrous cross-coupling conditions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)








![1-(4′-Methyl[1,1′-biphenyl]-4-yl)ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/224/118/064dfb31-1067-44ce-bf18-d3f762028eb6/640/064dfb31-1067-44ce-bf18-d3f762028eb6.png)
