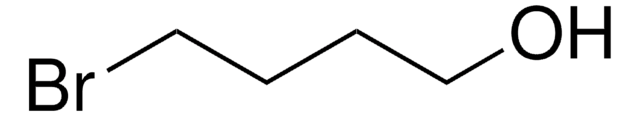
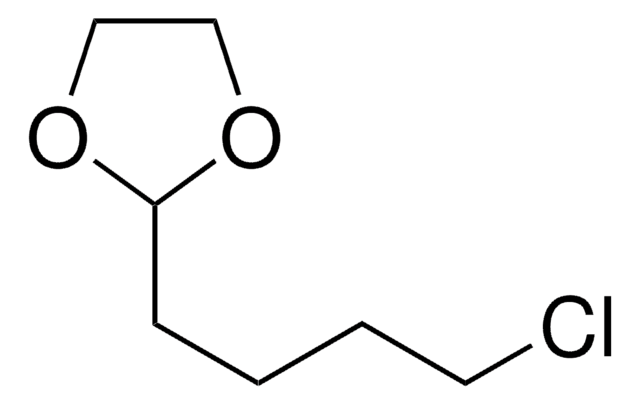
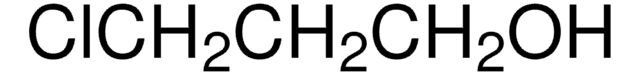26235
2-(3-Chloropropyl)-1,3-dioxolane
≥97.0% (GC)
Sinónimos:
4-Chlorobutyraldehyde ethylene acetal
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H11ClO2
Número de CAS:
Peso molecular:
150.60
Beilstein/REAXYS Number:
1236588
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥97.0% (GC)
form
liquid
refractive index
n20/D 1.453
bp
93-94 °C/12 mmHg (lit.)
density
1.142 g/mL at 20 °C (lit.)
functional group
chloro
ether
SMILES string
ClCCCC1OCCO1
InChI
1S/C6H11ClO2/c7-3-1-2-6-8-4-5-9-6/h6H,1-5H2
InChI key
ZBPUNVFDQXYNDY-UHFFFAOYSA-N
Categorías relacionadas
Application
2-(3-Chloropropyl)-1,3-dioxolane (2-(3′-chloropropyl)-1,3-dioxolane) is a masked γ-chlorobutyraldehyde and was used for the introduction of 3-(1,3-dioxolan-2-yl)propyl moiety. It was also used in the synthesis of:
- (±)-histrionicotoxin and (±)-histrionicotoxin 235A using a two-directional strategy
- 4-iodobutyraldehyde, 5-iodovaleraldehyde and 5-iodo-2-petanone
- corresponding phosphonate
Other Notes
Masked γ-chlorobutyraldehyde, useful for the introduction of the 3-(1,3-dioxolan-2-yl)propyl moiety; Preparation and use of the corresponding phosphonate
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
174.2 °F - closed cup
flash_point_c
79 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
R.E. Abbott et al.
The Journal of Organic Chemistry, 45, 5398-5398 (1980)
C.P. Forbes et al.
Journal of the Chemical Society. Perkin Transactions 1, 2353-2353 (1977)
A Nagy et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2464-2469 (1996-03-19)
A convenient, high yield conversion of doxorubicin to 3'-deamino-3'-(2''-pyrroline-1''-yl)doxorubicin is described. This daunosamine-modified analog of doxorubicin is 500-1000 times more active in vitro than doxorubicin. The conversion is effected by using a 30-fold excess of 4-iodobutyraldehyde in anhydrous dimethylformamide. The
P.A. Aristoff
The Journal of Organic Chemistry, 50, 1765-1765 (1985)
Two-directional synthesis. Part 1: A short formal synthesis of (?)-histrionicotoxin and (?)-histrionicotoxin 235A.
Stockman RA.
Tetrahedron Letters, 41(47), 9163-9165 (2000)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico