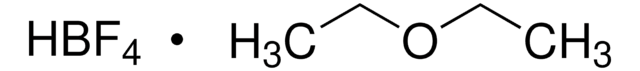175064
Nitrosyl tetrafluoroborate
95%
Sinónimos:
Nitrosonium tetrafluoroborate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
NOBF4
Número de CAS:
Peso molecular:
116.81
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
95%
reaction suitability
reagent type: oxidant
storage temp.
2-8°C
SMILES string
N#[O+].F[B-](F)(F)F
InChI
1S/BF4.NO/c2-1(3,4)5;1-2/q-1;+1
InChI key
KGCNVGDHOSFKFT-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Nitrosyl tetrafluoroborate is an efficient nitrosating and diazotizing agent. It reacts with alcohols and secondary amines to yield alkyl nitrites and nitrosamines, respectively. It reacts with primary amines to yield diazonium tetrafluoroborates. NOBF4 is also a mild oxidant and commonly used for single electron transfer oxidation.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
J Li et al.
Biochemical and biophysical research communications, 240(2), 419-424 (1997-12-06)
The caspases are a family of at least 10 human cysteine proteases that participate in cytokine maturation and in apoptotic signal transduction and execution mechanisms. Peptidic inhibitors of these enzymes are capable of blocking cytokine maturation and apoptosis, demonstrating their
S Mohr et al.
FEBS letters, 348(3), 223-227 (1994-07-18)
Previous studies have suggested that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) undergoes covalent modification of an active site thiol by a NO.-induced [32P]NAD(+)-dependent mechanism. However, the efficacy of GAPDH modification induced by various NO donors was found to be independent of spontaneous rates
S Mohr et al.
The Journal of biological chemistry, 274(14), 9427-9430 (1999-03-27)
S-Nitrosylation of protein thiol groups by nitric oxide (NO) is a widely recognized protein modification. In this study we show that nitrosonium tetrafluoroborate (BF4NO), a NO+ donor, modified the thiol groups of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by S-nitrosylation and caused enzyme
Electronically regulated thermally and light-gated electron transfer from anions to naphthalenediimides.
Guha, Samit et al.
Journal of the American Chemical Society, 133(39), 15256-15259 (2011)
C Würth et al.
Nanoscale, 9(12), 4283-4294 (2017-03-16)
The rational design of brighter upconversion nanoparticles (UCNPs) requires a better understanding of the radiationless deactivation pathways in these materials. Here, we demonstrate the potential of excitation power density (P)-dependent studies of upconversion (UC) luminescence intensities, slope factors, and absolute
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico