144932
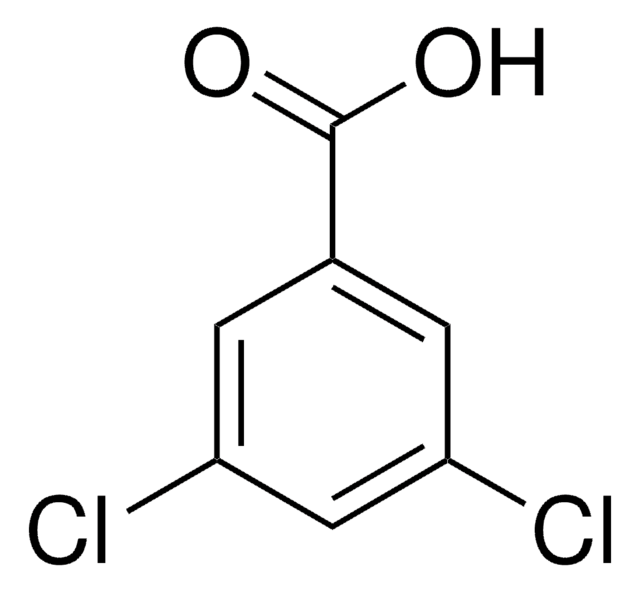
3,4-Dichlorobenzoic acid
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Cl2C6H3CO2H
Número de CAS:
Peso molecular:
191.01
Beilstein/REAXYS Number:
2044777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
solid
mp
204-206 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)c1ccc(Cl)c(Cl)c1
InChI
1S/C7H4Cl2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3H,(H,10,11)
InChI key
VPHHJAOJUJHJKD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
3,4-Dichlorobenzoic acid was employed as internal standard during the multiresidue analysis of pharmaceuticals and personal care products by ultra performance liquid chromatography-positive/negative electrospray tandem mass spectrometry. It was used to study the metabolic fate of 4-chloro-3,5-dinitrobenzoic acid.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Fate of substituted benzoates in the freshwater green alga, Chlamydomonas reinhardtii 11-32b.
Gutenkauf A, et al.
Biodegradation, 9(5), 359-368 (1998)
P Adriaens et al.
Applied and environmental microbiology, 57(1), 173-179 (1991-01-01)
When Acinetobacter sp. strain 4-CB1 was grown on 4-chlorobenzoate (4-CB), it cometabolized 3,4-dichlorobenzoate (3,4-DCB) to 3-chloro-4-hydroxybenzoate (3-C-4-OHB), which could be used as a growth substrate. No cometabolism of 3,4-DCB was observed when Acinetobacter sp. strain 4-CB1 was grown on benzoate.
Barbara Kasprzyk-Hordern et al.
Analytical and bioanalytical chemistry, 391(4), 1293-1308 (2008-02-07)
The main aim of the presented research is to introduce a new technique, ultra performance liquid chromatography-positive/negative electrospray tandem mass spectrometry (UPLC-ESI/MS/MS), for the development of new simultaneous multiresidue methods (over 50 compounds). These methods were used for the determination
K Umehara et al.
Drug metabolism and disposition: the biological fate of chemicals, 28(8), 887-894 (2000-07-20)
The metabolism of 1-(3,4-dichlorobenzyl)-5-octylbiguanide (OPB-2045), a new potent biguanide antiseptic, was investigated using rat and dog liver preparations to elucidate the mechanism of OPB-2045 metabolite formation, in which the octyl side chain is reduced to four, five, or six carbon
Yoshiteru Noutoshi et al.
Scientific reports, 2, 705-705 (2012-10-11)
Plant activators are agrochemicals that protect crops from pathogens. They confer durable resistance to a broad range of diseases by activating intrinsic immune mechanisms in plants. To obtain leads regarding useful compounds, we have screened a chemical library using an
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico