129445
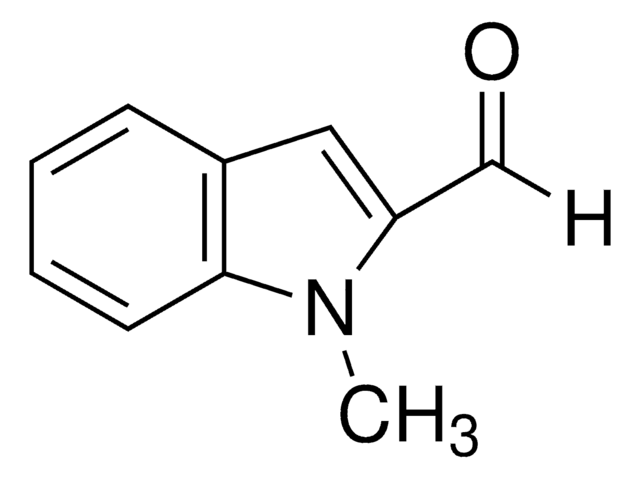
Indole-3-carboxaldehyde
97%
Sinónimos:
β-Indolylaldehyde, 3-Formylindole, 3-Indolylformaldehyde, Indole-3-carbaldehyde, NSC 10118
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H7NO
Número de CAS:
Peso molecular:
145.16
Beilstein/REAXYS Number:
114117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
solid
mp
193-198 °C (lit.)
functional group
aldehyde
SMILES string
O=Cc1c[nH]c2ccccc12
InChI
1S/C9H7NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-6,10H
InChI key
OLNJUISKUQQNIM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Indole-3-carboxaldehyde can undergo Schiff bases condensation to form multifunctional silica nano-vehicles and magnetic nanoparticles.
Application
Indole-3-carboxaldehyde was used to prepare analogs of the indole phytoalexin cyclobrassinin with NR1R2 group. It was also used as the starting material for the synthesis of higher order indoles including isoindolo[2,1-a]indoles, aplysinopsins, and 4-substituted-tetrahydrobenz[cd]indoles.
Reactant for preparation of:
- Analgesic agents
- Hypoglycemic agents
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Antibacterial and antifungal agents
- Antiamoebic and cytotoxic agents
- Inhibitors of the Dengue virus protease with antiviral activity in cell-culture
- Curcumin analogues as possible anti-proliferative & anti-inflammatory agents
- Inhibitors of Bcl-2 family proteins
- Inhibitors of the C-terminal domain of RNA Polymerase II as antitumor agents
- Inhibitors of TNF-α and IL-6 with anti-tubercular activity
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Qiu-Yun Chen et al.
Colloids and surfaces. B, Biointerfaces, 114, 158-163 (2013-11-05)
Multifunctional silica nano-vehicles (SiO2@indol-IL) and magnetic nanoparticles (Fe3O4@indol-IL) were constructed through the Schiff bases condensation of indole-3-carboxaldehyde and 4-acetyl-N-allyl pyridinium chloride (ILs) with the amine groups of silica and magnetic nanoparticles. SiO2@indol-IL can inhibit the proliferation of HepG-2 cells in
Indian J. Chem. B, 33, 4-4 (1994)
Heterocycles, 38, 1479-1479 (1994)
Mariana Budovská et al.
Bioorganic & medicinal chemistry, 21(21), 6623-6633 (2013-09-10)
An effective synthesis of analogs of the indole phytoalexin cyclobrassinin with NR1R2 group instead of SCH3 was developed starting from indole-3-carboxaldehyde. The target compounds were prepared by spirocyclization of 1-Boc-thioureas with the formation of isolable spiroindoline intermediates, followed by the
Tian-Lin Yang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 67(2), 568-571 (2006-09-22)
A new Schiff base ligand with tripodal structure, N,N',N''-tri-(3-indolemethanal)-triaminotriethylamine (L), and its complex with terbium was synthesized. The complex was characterized by element analysis, IR spectra, mass spectra, thermal analysis and molar conductivity. The terbium ion was found to coordinate
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico