109606
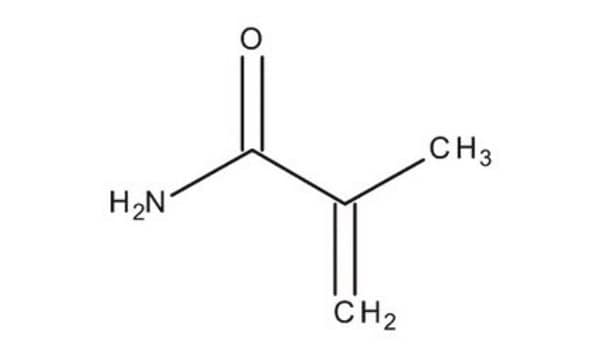
Methacrylamide
98%
Sinónimos:
2-Methylacrylamide, 2-Methylpropenamide, Methacrylic acid amide
About This Item
Productos recomendados
Ensayo
98%
Formulario
solid
mp
106-110 °C (lit.)
cadena SMILES
CC(=C)C(N)=O
InChI
1S/C4H7NO/c1-3(2)4(5)6/h1H2,2H3,(H2,5,6)
Clave InChI
FQPSGWSUVKBHSU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Synthesis of non-fouling poly brushes by photoinduced SET-LRP: This study highlights the use of photoinduced SET-LRP for the polymerization of methacrylamide, emphasizing its efficiency and the quality of the resulting polymers for non-fouling applications (M Vorobii, A de los Santos Pereira, 2015).
- Hydrolytic stability of methacrylamide and methacrylate in gelatin methacryloyl: The study investigates the hydrolytic stability of methacrylamide within gelatin methacryloyl, highlighting its stability and potential in biomedical applications (J Zheng, M Zhu, G Ferracci, NJ Cho, 2018).
- Two-step mechanisms of tumor selective delivery of N-(2-hydroxypropyl) methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage: This paper discusses the development of a copolymer conjugate for targeted cancer therapy, showcasing a two-step mechanism for enhanced drug delivery (H Nakamura, T Etrych, P Chytil, M Ohkubo, 2014).
- Backbone Degradable N-(2-Hydroxypropyl)methacrylamide Copolymer Conjugates with Gemcitabine and Paclitaxel: The research focuses on degradable copolymer conjugates for delivering cancer therapeutics, noting significant effects on tumor reduction and highlighting the impact of molecular weight (J Yang, R Zhang, H Pan, Y Li, Y Fang, 2017).
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 - STOT SE 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico