H4392
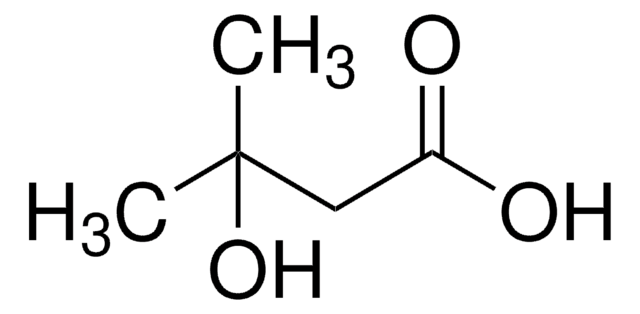
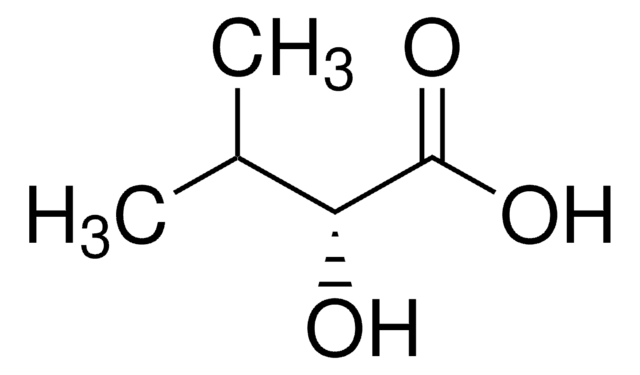
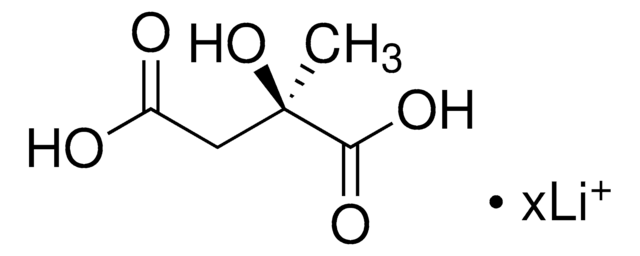
3-Hydroxy-3-methylglutaric acid
≥95%
Synonym(s):
3-Hydroxy-3-methylpentanedioic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC(CH3)(CH2CO2H)2
CAS Number:
Molecular Weight:
162.14
Beilstein:
1769194
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95%
mp
105-108 °C (lit.)
storage temp.
−20°C
SMILES string
CC(O)(CC(O)=O)CC(O)=O
InChI
1S/C6H10O5/c1-6(11,2-4(7)8)3-5(9)10/h11H,2-3H2,1H3,(H,7,8)(H,9,10)
InChI key
NPOAOTPXWNWTSH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Improvement of the functional value of green soybean (edamame) using germination and tempe fermentation: A comparative metabolomics study.: This research enhances the functional value of green soybean through germination and fermentation, identifying metabolites including those derived from 3-Hydroxy-3-methylglutaric acid. It offers insights into food processing and nutritional biochemistry (Iman et al., 2023).
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Beatriz Puisac et al.
Journal of inherited metabolic disease, 33(4), 405-410 (2010-06-10)
3-Hydroxy-3-methylglutaric aciduria is a rare human autosomal recessive disorder caused by deficiency of 3-hydroxy-3-methylglutaryl CoA lyase (HL). This mitochondrial enzyme catalyzes the common final step of leucine degradation and ketogenesis. Acute symptoms include vomiting, seizures and lethargy, accompanied by metabolic
S Funghini et al.
Molecular genetics and metabolism, 73(3), 268-275 (2001-07-20)
3-Hydroxy-3-methylglutaric aciduria is a rare autosomal recessive inborn error of metabolism caused by deficiency of the mitochondrial enzyme 3-hydroxy-3-methylglutaryl-CoA lyase (HMGCL). Up to now only a few mutations have been reported in the HMGCL gene. We report the first Italian
Elaine A Porter et al.
Phytochemistry, 81, 90-96 (2012-06-23)
LC-UV-MS/MS analysis of leaf extracts from 146 accessions of 71 species of Rosa revealed that some taxa accumulated flavonol O-glycosides acylated with 3-hydroxy-3-methylglutaric acid, which are relatively uncommon in plants. The structures of two previously unrecorded examples isolated from Rosa
Alister D Muir
Journal of AOAC International, 89(4), 1147-1157 (2006-08-19)
Flaxseed (Linum usitatissimum L.) is a major source of dietary intake of lignans by virtue of the high concentrations (0.7-1.5%) that are present in the seed. The principal lignan present in flaxseed is secoisolariciresinol diglucoside (SDG), which occurs as a
Jan Frank et al.
The British journal of nutrition, 92(1), 169-176 (2004-07-03)
Secoisolariciresinol diglucoside (SDG) is an important dietary lignan that is found at very high levels in flaxseed (1-4 %, w/w). Flaxseed lignans have received much research interest in recent years because of reported phyto-oestrogenic, anticarcinogenic, and anti-atherogenic effects. Previously, flaxseed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service