540234
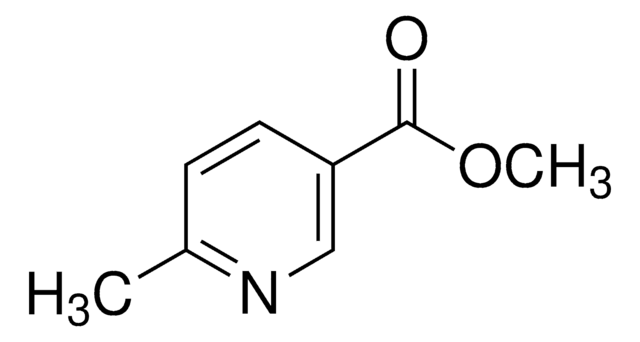
Dimethyl 2,5-pyridine dicarboxylate
97%
Synonym(s):
Methyl 6-methoxycarbonyl nicotinate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H9NO4
CAS Number:
Molecular Weight:
195.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
213-217 °C (lit.)
SMILES string
COC(=O)c1ccc(nc1)C(=O)OC
InChI
1S/C9H9NO4/c1-13-8(11)6-3-4-7(10-5-6)9(12)14-2/h3-5H,1-2H3
InChI key
TUGSJNQAIMFEDY-UHFFFAOYSA-N
General description
Dimethyl 2,5-pyridine dicarboxylate can be prepared from 2,5-pyridinedicarboxylic acid.
Application
Dimethyl 2,5-pyridine dicarboxylate (Dimethyl 2,5-pyridinedicarboxylate) may be used in the synthesis of:
- 2,5-dicarbomethoxy-N-methylpyridinium methosulfate
- 2,5-dicarboxy-N-methylpyridinium betaine
- 6-carbomethoxy-2-carboxypyridine-N-oxide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nitrogen-Substituted Derivatives of 2, 5-Pyridinedicarboxylic Acid.
Peterson ML.
The Journal of Organic Chemistry, 25(4), 565-569 (1960)
Marta Soler et al.
Inorganic chemistry, 54(22), 10542-10558 (2015-10-28)
The conjugation of redox-active complexes that can function as chemical nucleases to cationic tetrapeptides is pursued in this work in order to explore the expected synergistic effect between these two elements in DNA oxidative cleavage. Coordination complexes of biologically relevant
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service