All Photos(2)
About This Item
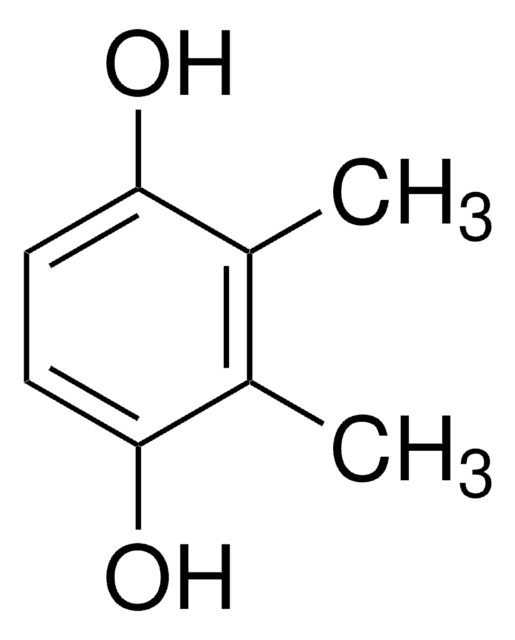
Linear Formula:
HOC6H3-1,3-(CO2H)2
CAS Number:
Molecular Weight:
182.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
298-302 °C (lit.)
SMILES string
OC(=O)c1cc(O)cc(c1)C(O)=O
InChI
1S/C8H6O5/c9-6-2-4(7(10)11)1-5(3-6)8(12)13/h1-3,9H,(H,10,11)(H,12,13)
InChI key
QNVNLUSHGRBCLO-UHFFFAOYSA-N
General description
The hydrothermal reaction of 5-hydroxyisophthalic acid, Co(II)/Cu(II) and dipyridophenazine, that forms two 3D chiral coordination polymers, was studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mathias Köberl et al.
Dalton transactions (Cambridge, England : 2003), 40(43), 11490-11496 (2011-09-29)
The treatment of the dimeric paddle-wheel (PW) compound [Mo(2)(NCCH(3))(10)][BF(4)](4)1 with oxalic acid (0.5 equiv.), 1,1-cyclobutanedicarboxylic acid (1 equiv.), 5-hydroxyisophthalic acid (1 equiv.) (m-bdc-OH) or 2,3,5,6-tetrafluoroterephthalic acid (0.5 or 1 equiv.) leads to the formation of macromolecular dicarboxylate-linked (Mo(2))(n) entities (n
Mamoru Yamada et al.
Biotechnology letters, 32(3), 445-450 (2009-11-27)
5-Hydroxyisophthalic acid-producing microorganisms were isolated from enrichment cultures using 5-sulfoisophthalic acid as a sulfur source. One bacterium, Ochrobactrum anthropi S9, had the highest 5-sulfoisophthalic acid-degrading activity, and stoichiometrically formed 5-hydroxyisophthalic acid, a raw material for polymer synthesis. Under optimum culture
Zheng-Bo Han et al.
Dalton transactions (Cambridge, England : 2003), (44)(44), 9807-9811 (2009-11-04)
The hydrothermal reaction of Co(II)/Cu(II), 5-hydroxyisophthalic acid and dipyridophenazine leads to the generation of two 3D chiral coordination polymers, [M(hip)(DPPZ)](n) (M = Co(), Cu(), H(2)hip = 5-hydroxyisophthalic acid, DPPZ = dipyridophenazine), which contain M-hip-M helical chains (M = Co, Cu)
Kaisa Hänninen et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 31(5), 306-317 (2007-06-22)
Donnan theory was applied to gain mechanistic understanding on the factors affecting drug loading process, compound-fiber affinity and subsequent release from fibrous ion-exchangers. Impact of initial loading solution concentration on fiber occupancy and loading efficiency of compounds were assessed experimentally
Jiyoung Lee et al.
Nature communications, 8, 14070-14070 (2017-01-05)
Self-assembly has proven to be a widely successful synthetic strategy for functional materials, especially for metal-organic materials (MOMs), an emerging class of porous materials consisting of metal-organic frameworks (MOFs) and metal-organic polyhedra (MOPs). However, there are areas in MOM synthesis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service