D1315
DT Diaphorase (NQO1) human
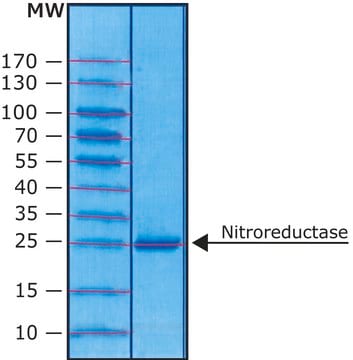
lyophilized powder, recombinant, expressed in E. coli, ≥90% (SDS-PAGE)
Synonym(s):
DTD, NQO1, Quinone reductase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli
Quality Level
Assay
≥90% (SDS-PAGE)
form
lyophilized powder
specific activity
≥100 units/mg protein
mol wt
monomer 31000
UniProt accession no.
storage temp.
2-8°C
Gene Information
human ... NQO1(1728)
Application
Human DT diaphorase has been used in a study to assess the development of novel quinone phosphorodiamidate prodrugs. Human DT diaphorase has also been used to investigate its crystal structure for the development of a model for its interaction with the cytotoxic prodrug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954).
Biochem/physiol Actions
DT-diaphorase, also referred to as NAD(P)H:(quinone-acceptor) oxidoreductase, is involved in the reductive activation process of several cytotoxic antitumor quinones and nitrobenzenes. It catalyzes the two-electron reduction of quinones and quinonoid compounds to hydroquinones, using either NADH or NADPH as the electron donor. The flavoenzyme contains one mole of FAD per mole of enzyme.
NQO1 is a cytosolic homodimeric FAD-dependent enzyme that catalyses the reduction of a broad range of cytotoxic quinones resulting in protection from cellular oxidative stress. Oxidative stress may also enhance NQO1-mediated protection of p53 and p73 against proteasomal degredation. The highly Inducible expression of NQO1 is controlled by the Nrf2-Keap1/ARE pathway and appears to be affected by changes in susceptibility to oxidative stress. During Oxygen/glucose deprivation, NQO1 appears to be involved in AMPK-induced cancer cell death. NQO1 has been observed to be overexpressed in several types of solid tumors, including breast, pancreas, lung and colon cancer.
Shown to activate quinone based anti-tumor agents in vivo. Suitable for conjugation to carrier molecules.
Unit Definition
One unit will reduce 1.0 μmole cytochrome C per min/mg in the presence of menadione substrate at 37 °C.
inhibitor
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Molecular of Modelling of Human DT-Diaphorase for Enzyme-Directed Bioreductive Drug Design.
Doughtyl, S.W. and Phillips, R.M.
Molecular Simulations, 24, 209-209 (2000)
Yang Yang et al.
Journal of experimental & clinical cancer research : CR, 33, 14-14 (2014-02-07)
NAD (P) H: quinone oxidoreductase 1 (NQO1) is a xenobiotic metabolizing enzyme that detoxifies chemical stressors and antioxidants, providing cytoprotection in normal tissues. However, high-level expression of NQO1 has been correlated with numerous human malignancies, suggesting a role in carcinogenesis
C Flader et al.
Journal of medicinal chemistry, 43(16), 3157-3167 (2000-08-24)
A series of naphthoquinone and benzimidazolequinone phosphorodiamidates has been synthesized and studied as potential cytotoxic prodrugs activated by DT-diaphorase. Reduction of the quinone moiety in the target compounds was expected to provide a pathway for expulsion of the phosphoramide mustard
S Chen et al.
The Journal of biological chemistry, 272(3), 1437-1439 (1997-01-17)
DT-diaphorase (EC 1.6.99.2), also referred to as NAD(P)H:(quinone-acceptor) oxidoreductase, is involved in the reductive activation process of several cytotoxic antitumor quinones and nitrobenzenes. It has been observed in our and other laboratories that the rat enzyme is significantly more effective
David Siegel et al.
Frontiers in pharmacology, 13, 1015642-1015642 (2022-11-22)
The stress induced protein NQO1 can participate in a wide range of biological pathways which are dependent upon the interaction of NQO1 with protein targets. Many of the protein-protein interactions involving NQO1 have been shown to be regulated by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service