C9409
Creatinine Deiminase microbial
lyophilized powder, ≥25 units/mg protein
Synonym(s):
Creatinine Deaminase
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
lyophilized powder
specific activity
≥25 units/mg protein
mol wt
~260 kDa
composition
Protein, ≥15% biuret
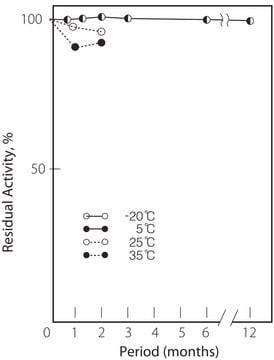
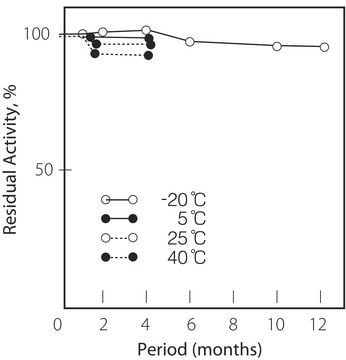
storage temp.
−20°C
Application
Creatinine Deiminase microbial has been used:
- to immobilize aminosilylated glass beads based biosensor for ammonia/ammonium and creatinine detection in urine
- in creating creatinine-sensing membrane for biophysical studies
- to investigate the bioelectronic tongue for the simultaneous determination of urea, creatinine and alkaline ions in clinical samples
Creatinine deiminase has been used in a study to assess the application of a creatinine-sensitive biosensor for hemodialysis control. Creatinine deiminase has also been used in a study to investigate the bioelectronic tongue for the simultaneous determination of urea, creatinine and alkaline ions in clinical samples.
Biochem/physiol Actions
Creatinine deiminase catalyzes the hydrolysis of creatinine to methylhydantoine and ammonia.
Physical properties
Isoelectric point : 4.4
Michaelis constant : 3.5 x 10‾3M (Creatinine)
Structure : 6 subunits per mol of enzyme
Inhibitors : Ag+,Hg++, o-phenanthroline,monoiodoacetate
Optimum pH : 8.5 – 9.5
Optimum temperature : 65 – 75°C
pH Stability : pH 7.0 – 11.0 (30°C, 20hr)
Thermal stability : Below 65°C (pH 7.5, 1hr)
Michaelis constant : 3.5 x 10‾3M (Creatinine)
Structure : 6 subunits per mol of enzyme
Inhibitors : Ag+,Hg++, o-phenanthroline,monoiodoacetate
Optimum pH : 8.5 – 9.5
Optimum temperature : 65 – 75°C
pH Stability : pH 7.0 – 11.0 (30°C, 20hr)
Thermal stability : Below 65°C (pH 7.5, 1hr)
Unit Definition
One unit will hydrolyze 1.0 μmole of creatinine to N-methylhydantoin and NH3 per min at pH 7.5 at 37 °C in a coupled system with L-glutamic dehydrogenase.
Physical form
Lyophilized powder containing mannitol as stabilizer
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Z K He et al.
Analytical biochemistry, 283(2), 166-174 (2000-07-25)
A precise and sensitive working microflow titration procedure was developed to determine creatinine and ammonia in urine samples. This procedure is based on enzymatic conversion of creatinine, gas diffusional membrane separation of the released ammonia into an acid acceptor stream
R G Washburn et al.
The Journal of antimicrobial chemotherapy, 17(5), 673-677 (1986-05-01)
Creatinine iminohydrolase (EC 3.5.4.21) quantitatively releases ammonia from flucytosine (5FC) as well as from creatinine. Using 39 sera from eight patients receiving the combination of amphotericin B with 5FC, we demonstrated that this rapid enzymatic reaction provides a valid measure
Manuel Gutiérrez et al.
Biosensors & bioelectronics, 23(6), 795-802 (2007-10-13)
Urea and creatinine biosensors based on urease and creatinine deiminase, respectively, covalently immobilized onto ammonium selective electrodes, were included in an array together with sensors sensitive to ammonium, potassium and sodium. Generic sensors to alkaline ions were also included. All
C M Huang et al.
Clinical chemistry, 34(1), 59-62 (1988-01-01)
We developed an enzymatic method for determination of 5-fluorocytosine in serum, using creatine iminohydrolase (EC 3.5.4.21), the Cobas-Bio analyzer, and an extant ammonia method. Analytical recovery (y) of drug added to serum (x) was good, with y = 0.97x-0.7, Sy.x
Anne K Bendt et al.
Archives of microbiology, 181(6), 443-450 (2004-05-19)
In order to utilize different nitrogen sources and to survive situations of nitrogen limitation, microorganisms have developed several mechanisms to adapt their metabolism to changes in the nitrogen supply. In this communication, the use of creatinine as an alternative nitrogen
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service