F13207
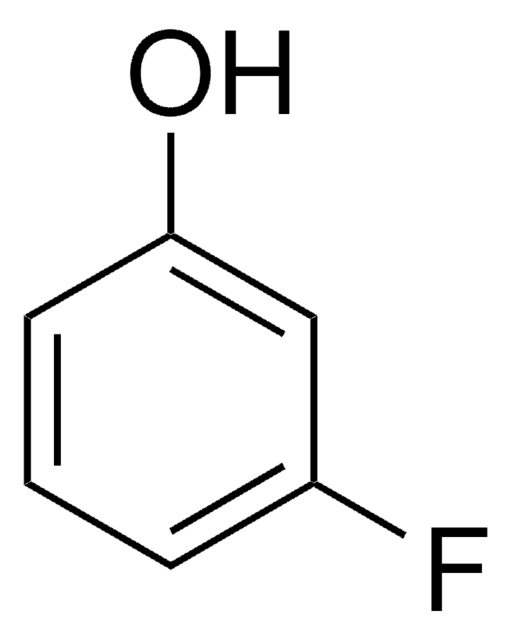
4-Fluorophenol
99%
Synonym(s):
4-Hydroxyphenyl fluoride, p-Fluorophenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4OH
CAS Number:
Molecular Weight:
112.10
Beilstein:
1362752
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
185 °C (lit.)
mp
43-46 °C (lit.)
SMILES string
Oc1ccc(F)cc1
InChI
1S/C6H5FO/c7-5-1-3-6(8)4-2-5/h1-4,8H
InChI key
RHMPLDJJXGPMEX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A M Osman et al.
Chemico-biological interactions, 104(2-3), 147-164 (1997-05-02)
The present study shows that MP8 in the presence of H2O2 is able to catalyze the rupture of the stable carbon-fluorine bond of 4-fluorophenol, used as a model substrate for the oxidative dehalogenation reaction. 1,4-Benzoquinone was shown to be the
Alexandria Harkey et al.
Biotechnology letters, 34(9), 1725-1731 (2012-05-29)
Cytochrome P450(BM3)-F87G catalyzed the oxidative defluorination of 4-fluorophenol, followed by reduction of the resulting benzoquinone to hydroquinone via the NADPH P450-reductase activity of the enzyme. The k (cat) and K (m) for this reaction were 71 ± 5 min(-1) and
M Esseffar et al.
The journal of physical chemistry. A, 113(52), 14711-14717 (2009-10-15)
The interaction of 3,4 dinitrophenol (DNP) with cyclic ketones, lactones, and lactams was investigated by UV-visible spectroscopy and density functional theory (DFT) methods. Equilibrium constants K(HB) for 1:1 hydrogen bonded complexes were determined in solution in CCl(4) and C(6)H(12). For
Manfred Lehnig et al.
Organic & biomolecular chemistry, 4(4), 721-729 (2006-02-10)
Peroxynitric acid (O2NOOH) nitrates L-tyrosine and related compounds at pH 2-5. During reaction with O2(15)NOOH in the probe of a 15N NMR spectrometer, the NMR signals of the nitration products of L-tyrosine, N-acetyl-L-tyrosine, 4-fluorophenol and 4-methoxyphenylacetic acid appear in emission
Thomas Ludwig et al.
Nuclear medicine and biology, 29(2), 255-262 (2002-02-02)
This paper describes the improved synthesis of n.c.a. 4- 18F]fluorophenol for the preparation of 18F-labeled alkylarylethers. Nucleophilic fluorination of substituted benzophenone derivatives yielded n.c.a. 4- 18F]fluoro-4'-substituted benzophenones with 80- 90% RCY, which were converted to benzoic acid phenylesters by treatment
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service